 Open Access Article
Open Access ArticleThe beneficial effect of protic ionic liquids on the lithium environment in electrolytes for battery applications
Thomas
Vogl
,
Sebastian
Menne
,
Ruben-Simon
Kühnel
and
Andrea
Balducci
*
Westfälische Wilhelms-Universität, Institut für Physikalische Chemie-MEET, Corrensstraße 28/30, 48149 Münster, Germany. E-mail: andrea.balducci@uni-muenster.de
First published on 13th March 2014
Abstract
This work reports the improved rate performance of ionic-liquid based lithium-ion batteries by replacement of the conventional aprotic ionic liquid (AIL) N-butyl-N-methylpyrrolidinium bis(trifluoromethanesulfonyl)imide (PYR14TFSI) by protic ionic liquids (PILs). Two model pyrrolidinium-TFSI PILs are synthesized and their mixtures with lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) are characterized in terms of conductivity, viscosity and self-diffusion coefficients. Raman measurements show pronounced differences in terms of TFSI− coordination to Li+ between the AIL and the PILs. Li+ is coordinated by significantly fewer TFSI− anions in the investigated PILs, which is discussed as the likely cause for the much improved rate performance of lithium vanadium phosphate-based electrodes in these electrolytes.
1. Introduction
Lithium-ion batteries (LIBs) are nowadays one of the most important energy storage devices. LIBs are already dominating the consumer portable electronics market and have been indicated as the most promising electrochemical devices for the realization of hybrids and electric vehicles, as well as for advanced delocalized energy storage units. However, in order to be increasingly used in these new applications, the performance and safety of LIBs need to be improved.1The commercially available LIBs contain electrolytes based on organic carbonates (e.g. ethylene carbonate (EC) and diethyl carbonate (DEC)). These electrolytes feature good conductivities, but since they are easily flammable and volatile, their use strongly reduces the safety as well as the temperature range of use of LIBs. For these reasons, in the last few years tremendous efforts have been focused on the development of alternative electrolytes.1
Ionic liquids (ILs) are presently considered as one of the most promising candidates for the replacement of organic carbonates.2–4 The main advantages of ILs towards organic carbonates are the high thermal, chemical and electrochemical stability as well as the negligible vapor pressure and the reduced flammability.2 So far, several aprotic ionic liquids (AILs) have been investigated as electrolytes for LIBs. The results of these studies showed that AILs can be successfully introduced in LIBs, and their use has beneficial effects on the safety as well as on the temperature range of use of these devices.3 Nevertheless, AILs are rather expensive and the performance of AIL-based LIBs still needs to be improved in order to be fully competitive with that of conventional electrolytes. Such improvement is particularly necessary for applications where high current densities are needed. In these applications the reduced lithium-ion transport of AIL-based electrolytes, compared to that of conventional electrolytes, might significantly affect the rate performance of LIBs. In order to overcome this limitation, the use of mixtures of ionic liquids and organic electrolytes appears as a promising strategy.3,5,6 Nevertheless, since such mixtures contain organic solvents, their safety is still lower compared to that of electrolytes containing only ILs.5,6 For these reasons, the development of new IL-based electrolytes with improved lithium-ion mobility compared to the one of present AIL-based electrolytes nowadays appears to be important for the introduction of ILs in LIBs. Taking into account the high cost of AILs, it would also be very beneficial to develop cheaper ILs, as they could be easily introduced in commercial devices.
Protic ionic liquids (PILs) are a subset of ILs and they are typically synthesized by neutralization reactions of a Brønsted acid (proton donor) and a Brønsted base (proton acceptor).7 PILs display all favorable properties of ILs, but they have the advantage of being easier to synthesize and cheaper compared to AILs.7,8 Clearly, these properties make them interesting candidates for use as electrolyte components for electrochemical devices. So far PILs have been successfully introduced as electrolytes for fuel cells and supercapacitors.8–11 In the case of fuel cells, PILs appear as interesting candidates for non-humidifying intermediate-temperature fuel cells operating at a relatively high temperature.11 In the case of supercapacitors, a recent study showed that the use of PILs allows the realization of devices with stable performance at different temperatures, but with lower operating voltage compared to that of conventional systems.10
It is worth noticing that the use of PILs as electrolytes for LIBs was not considered in the past. The availability of an acidic proton and its strong reactivity towards lithium were seen as an obstacle for the introduction of PILs into these devices, and consequently all efforts were focused on AILs. Nevertheless, we recently showed that in dry PILs the labile proton of the cation is not “free” and this cation is not subject to reversible protonation–deprotonation.10 We also proved that battery materials, e.g. lithium iron phosphate (LFP), can be used in combination with dry PILs without being subject to structural changes.12 Moreover, we showed that lithium-ion batteries containing PIL-based electrolytes can be realized and that they display promising performance in terms of capacity and cycling stability.13 Taking into account these results, PILs can therefore be regarded as a new class of electrolytes for LIBs. However, since only few studies have been dedicated to these electrolytes, a deeper investigation of PIL-based electrolytes is needed to understand the advantages and the limits related to their use in LIBs.
In this manuscript we report about the use of two pyrrolidinium-based PILs, pyrrolidinium bis(trifluoromethanesulfonyl)imide (PYRHHTFSI) and N-butylpyrrolidinium bis(trifluoromethanesulfonyl)imide (PYRH4TFSI), in combination with lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) as electrolytes for lithium-ion batteries. Initially the thermal stability, viscosity and conductivity of the two PIL-based electrolytes were considered. Afterwards, with the goal to get new insights into this class of electrolytes, the lithium mobility of these PIL-based electrolytes was investigated for the first time. Finally, the use of PIL-based electrolytes in combination with lithium vanadium phosphate (LVP) was evaluated. For all investigations, a comparison with the behavior of a pyrrolidinium-based AIL, 0.5 M LiTFSI in N-butyl-N-methylpyrrolidinium bis(trifluoromethanesulfonyl)imide (PYR14TFSI), was made.
2. Experimental section
The pyrrolidinium-based PILs, PYRHHTFSI and PYRH4TFSI, were synthesized following a procedure similar to that described elsewhere.14 Pyrrolidine (Aldrich, >99%) and 1-butylpyrrolidine (Aldrich, >98%) were distilled directly before use. HCl (37%) and LiTFSI (3 M) were used as received. At the end of the synthesis, the obtained PILs were dried under vacuum at 60 °C. The water content of the PILs was measured using coulometric Karl-Fischer titration, and was found to be lower than 10 ppm.Thermogravimetric analysis was carried out using a Q5000 TGA instrument (TA Instruments). Ionic liquid samples with a typical weight of 20–30 mg were used during the experiments. The samples were heated from room temperature to 600 °C with a heating rate of 5 °C min−1 using nitrogen as a purge gas (10 cm3 min−1).
The conductivity and the viscosity of the prepared ILs were determined as reported in ref. 12. Self-diffusion coefficients were determined at 30 °C by pulsed field gradient (PFG)-NMR with an Avance III spectrometer (Bruker) equipped with a Diff30 probehead (Bruker) with selective RF-inserts for 1H, 7Li and 19F using the pulsed gradient stimulated echo (diffSte) sequence.15 Gradient pulse length, minimum gradient strength and diffusion time were set to 1 ms, 10 Gs cm−1 and 100 ms for the 1H and 19F measurements and 3 ms, 20 Gs cm−1 and 70 ms for the 7Li measurements. The maximum gradient strength was chosen in order to obtain a strong signal damping. A typical maximum gradient strength was 800 Gs cm−1. The fitting was done with TopSpin 3.0 (Bruker) software using the Stejskal–Tanner equation. All proton signals were considered for the determination of the pyrrolidinium diffusion coefficients.
Raman spectra were recorded with a software-controlled Bruker Vertex 70 RAM II FT-Raman device with a HeNe-laser, a CaF2 beam splitter and a LN-Ge diode as a detector. The number of scans was set to 512 and the laser power was set to 500 mW. The Raman spectra were recorded at room temperature.
Lithium vanadium phosphate (LVP) composite electrodes were prepared as in ref. 16. The composition of the electrodes was 70 wt% of the active material LVP, 20 wt% of the conductive agent Super C65® and 10 wt% of polyvinylidene fluoride (PVDF) as a binder. The electrode mass loading was 1 mg cm−2 and the electrode area was 1.13 cm2. All the electrochemical tests were carried out with 3-electrode Swagelok® type cells. The cells were assembled in an argon-filled glove box with oxygen and water contents lower than 1 ppm. LVP-based electrodes were used as working electrodes, a silver wire was used as the pseudo-reference electrode and an oversized activated carbon-based electrode was used as the counter electrode. For all experiments, a 3-layered non-woven separator (Freudenberg) drenched with 100 μL of electrolyte was used.
Electrochemical impedance spectroscopy (EIS) was performed at 30 °C using a VMP multichannel potentiostatic–galvanostatic system (Biologic Science Instruments, France). Prior to the measurements, 20 galvanostatic cycles with a current corresponding to a rate of 1 C were carried out. Afterward, impedance spectra were recorded at the fully lithiated state (fully discharged) and in a partially lithiated state (at the first plateau of the discharge) in the frequency range between 1 MHz and 10 mHz with an alternating voltage of 5 mV.
The electrochemical measurements were carried out using a MACCOR Series 4000 battery tester. Constant current cycling (CC) was carried out at 30 °C using current densities ranging from 1 C to 20 C taking into account the theoretical capacity of LVP of 131 mA h g−1 when cycled between 3.0 and 4.3 V vs. Li/Li+.
3. Results and discussion
The chemical–physical properties of ILs are dramatically affected by the presence of impurities and the presence of water.2–4,13 As mentioned in the Introduction, a pre-requisite for the utilization of PILs in LIBs is the use of dry electrolytes (with water content lower than 20 ppm) as under these conditions the labile proton(s) of the cation is not “free” and it does not interfere with the lithiation–delithiation process of the LIB electrodes.10,13 As indicated in the Experimental section, the investigated PILs displayed water contents below 10 ppm, and therefore they both appear suitable for use as electrolytes for LIBs.3.1. Thermal stability
Fig. 1 compares the thermal stability of the PIL-based electrolytes, 0.5 M LiTFSI in PYRHHTFSI and 0.5 M LiTFSI in PYRH4TFSI, with that of the AIL-based electrolyte 0.5 M LiTFSI in PYR14TFSI. LiTFSI was selected as lithium salt since it has the same anion of all ILs. As shown, both PIL-based electrolytes are thermally stable up to 250 °C. Between the two, the electrolyte containing PYRHHTFSI appears slightly more stable than the one containing PYRH4TFSI. The electrolyte containing the AIL PYR14TFSI, on the other hand, appears more stable as a significant weight loss begins at more than 300 °C. These results clearly indicate that PIL-based electrolytes display thermal stability significantly higher than that of conventional organic electrolytes.6 | ||
| Fig. 1 Thermal stability of the electrolytes 0.5 M LiTFSI in PYRHHTFSI, 0.5 M LiTFSI in PYRH4TFSI and 0.5 M LiTFSI in PYR14TFSI. | ||
3.2. Conductivity and viscosity
Fig. 2 compares the conductivity and viscosity of two PIL-based electrolytes, 0.5 M LiTFSI in PYRHHTFSI and 0.5 M LiTFSI in PYRH4TFSI, with those of the AIL-based electrolyte 0.5 M LiTFSI in PYR14TFSI. As shown in Fig. 2a, the two PIL-based electrolytes display similar conductivity in the investigated temperature range. At 30 °C they both display conductivity in the order of 2 mS cm−1, which increases to 7 mS cm−1 at 80 °C. These values are comparable with the ones shown by the electrolyte 0.5 M LiTFSI in PYR14TFSI in the same temperature range. It is worth noticing that it is often reported in the literature that PILs display higher conductivity compared to AILs.17 Nevertheless, most of the reported values refer to PILs containing high amounts of water and, most likely, the presence of water is the origin of this high conductivity.13 As shown above, when dry PILs are considered a marked difference in terms of ionic conductivity between PILs and AILs cannot be observed. Fig. 2b shows the variation of the viscosity over the temperature for two PIL-based electrolytes. As for the conductivity, also the viscosity of the PIL-based electrolytes was comparable to that of 0.5 M LiTFSI in PYR14TFSI. Nevertheless, it is interesting to note that at 30 °C the electrolyte 0.5 M LiTFSI in PYRHHTFSI displays a viscosity of 130.1 mPa s, which is higher than the one of 0.5 M LiTFSI in PYRH4TFSI (96.0 mPa s). Taking into account the conductivities of the two electrolytes, the higher viscosity of PYRHHTFSI compared to PYRH4TFSI appears somehow surprising. This behavior might be originated from the different size of the cations of these PILs as well as from their different interactions with the TFSI− anion. The PYRHH+ cation is obviously smaller than the PYRH4+ cation and this characteristic might be responsible for the higher conductivity of the electrolyte containing PYRHHTFSI compared to the one containing PYRH4TFSI. At the same time, as it is less shielded, the cation PYRHH+ is probably subject to a stronger interaction with the anion TFSI− compared to the cation PYRH4+, and this interaction might explain the higher viscosity of the electrolyte containing PYRHHTFSI compared to the one containing PYRH4TFSI. At higher temperatures, the viscosity of both electrolytes decreases and becomes more similar. At 80 °C, both electrolytes have viscosities in the range of 14.4–18.0 mPa s. | ||
| Fig. 2 Conductivity (a) and viscosity (b) of the electrolytes 0.5 M LiTFSI in PYRHHTFSI, 0.5 M LiTFSI in PYRH4TFSI and 0.5 M LiTFSI in PYR14TFSI. | ||
The temperature dependence of the measured conductivities and viscosities did not show Arrhenius-like behavior, but it could be well described by the Vogel–Tammann–Fulcher (VTF) model. Fig. 3 shows the VTF plots for conductivity and viscosity of all electrolytes. The VTF-equations (1) and (2) are shown below and the VTF fitting parameters are reported in Table 1.
 | (1) |
 | (2) |
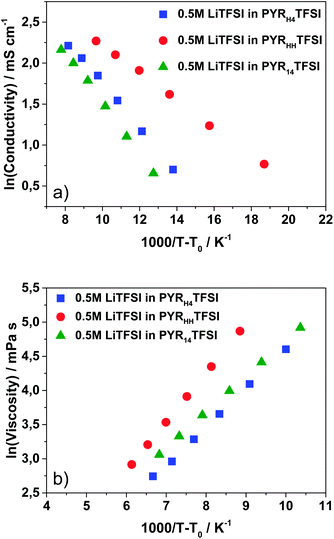 | ||
| Fig. 3 VTF plots of conductivity (a) and viscosity (b) of the electrolytes 0.5 M LiTFSI in PYRHHTFSI, 0.5 M LiTFSI in PYRH4TFSI and 0.5 M LiTFSI in PYR14TFSI. | ||
| Conductivity | Viscosity | |||||
|---|---|---|---|---|---|---|
| T 0c/K | σ 0/mS cm−1 | B c/K | T 0v/K | η 0/mPa s | B v/K | |
| PYRH4TFSI | 230.5 | 49.49 | 168.0 | 203.0 | 0.356 | −562.7 |
| PYRHHTFSI | 249.5 | 84.64 | 268.9 | 190.0 | 0.225 | −718.5 |
| PYR14TFSI | 224.5 | 95.94 | 305.0 | 206.5 | 0.587 | −526.9 |
3.3. Diffusion measurements
As shown above, the conductivity and viscosity of dry pyrrolidinium-based PILs are rather similar to those of pyrrolidinium-based AILs. As mentioned in the Introduction, one of the main limitations of AIL-based electrolytes is their reduced lithium-ion mobility compared to the one of conventional electrolytes. The ion mobility as well as the lithium coordination of AILs has already been investigated in the past.18,19 Nevertheless, to the best of our knowledge, so far the Li+ mobility as well as the lithium coordination in PIL-based electrolytes has not been investigated. Considering the importance of these properties for the development of advanced IL-based electrolytes for LIBs, we therefore investigated both of them. Table 2 lists self-diffusion coefficients and the dissociation degree for the investigated electrolytes. Only relatively small differences in self-diffusion coefficients between the different electrolytes were found. This was surprising; it was expected that reducing the size of the pyrrolidinium cation by substituting one or both of the alkyl chains of PYR14+ with a hydrogen atom would accelerate its diffusion, in line with the increasing conductivity (Fig. 2a). Substituting the methyl group of PYR14+ by a hydrogen atom had the opposite effect: all self-diffusion coefficients became slightly smaller, although the conductivity of this electrolyte was found to be higher than the one of PYR14TFSI–0.5 M LiTFSI. This can be understood by considering that it cannot be distinguished between un-paired and aggregated ions with the NMR measurements. Hence, also ion pairs and other neutral aggregates can contribute to the measured self-diffusion coefficients. These neutral species do not contribute to the electrolyte conductivity, which is consequently lowered by their presence. The Nernst–Einstein equation (eqn (3)) allows the calculation of an apparent conductivity treating all diffusing species as charge carriers contributing to conductivity: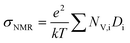 | (3) |
3.4. Lithium-ion environment
To investigate the different ion–ion interactions in AIL- and PIL-based electrolytes in more detail, Raman spectra were recorded for all electrolytes. A characteristic (and strong) signal relative to the interaction of TFSI− with other ions is the mode, found at a wavenumber of 742 cm−1, which is generated by the expansion/contraction of the whole TFSI− anion. In the literature, this signal is typically assigned to non-coordinating or “free” TFSI− anions.20–22 Coordinating (or interacting) TFSI− anions generate an additional signal, which is shifted to higher wavenumbers in the Raman spectra (e.g. lithium ion coordinating TFSI−, ca. 748 cm−1).23,24Table 3 lists the assigned peak positions for the two modes (non-coordinating/coordinating) observed in the investigated electrolytes. The non-coordinating TFSI− (TFSInon) mode for 0.5 M LiTFSI in PYR14TFSI was found to be at 742.4 cm−1. This value is in agreement with values reported in the literature for “free” TFSI-anions.23 In the case of the PIL-based electrolytes, the TFSInon mode was shifted to higher wavenumbers, indicating the presence of relatively important ion–ion interactions between the IL-cations and TFSI− in these electrolytes. The presence of these interactions is also confirmed by the shift of the peak wavenumbers corresponding to the Li+ coordinating TFSI− (TFSIcoor) to higher wavenumbers, which is more marked in the case of the two PIL-based electrolytes than for the electrolytes containing PYR14TFSI (Fig. 4). In order to evaluate the ratio between coordinated and free ionic species, a useful method is to compare the areas of these species in the Raman spectra.24| Ionic liquid | TFSInon/cm−1 | TFSIcoor/cm−1 | TFSIcoor/% | n in (Li+) (TFSI−)n |
|---|---|---|---|---|
| PYR14TFSI | 742.4 | 748.5 | 27.04 | 1.9 |
| PYRH4TFSI | 744.6 | 749.0 | 16.47 | 1.2 |
| PYRHHTFSI | 745.6 | 749.7 | 9.12 | 0.4 |
 | ||
| Fig. 4 Raman spectra in the spectral range from 730–760 cm−1 of 0.5 M LiTFSI in PYR14TFSI (a), PYRH4TFSI (b) and PYRHHTFSI (c). | ||
Fig. 4 shows the Raman spectra of the three electrolytes and the fitted areas for non-coordinating and coordinating TFSI−. From the figures it can be seen that the areas of TFSIcoor became smaller moving from PYR14TFSI to PYRH4TFSI to PYRHHTFSI. Accordingly, the fraction of TFSIcoor decreases (as indicated in Table 3), following the same trend. When the lithium coordination number in the investigated electrolyte is calculated,19 the different behavior of PILs and AILs became evident. As indicated in Table 3, in PYR14TFSI the Li+ ions are well coordinated by ca. 2 TFSI− anions, while in PYRHHTFSI the Li+ ions are coordinated by only 0.4 TFSI− anions. Taking this result into account, it is reasonable to suppose that the less shielded PIL-cations restrain TFSI− anions from coordinating to Li+ ions. As a consequence of this “cation competition”, the Li+ ion in PIL-based electrolytes becomes significantly less coordinated compared to AILs.25
The results reported above clearly indicate that AIL- and PIL-based electrolytes, although they display similar ionic conductivity and viscosity, display marked differences in terms of ion coordination. As shown by the Raman study, the interaction between Li+ and TFSI− is much stronger in the AIL-based electrolyte than in the case of the PIL-based ones. The lower interaction in the latter electrolytes is due to the presence of less shielded cations, which “compete” with the Li+ ion for the coordination of the anion. Nevertheless, it is very interesting to notice that the differences in coordination did not lead to pronounced changes in the lithium diffusion coefficient, as indicated by the NMR measurement. Taking these findings into account, the main difference between AIL- and PIL-based electrolytes appears to be the lithium environment (see Fig. 4). Li+ is certainly more loosely coordinated in the investigated PILs than in the investigated AILs. This difference appears extremely interesting as it could have a positive impact on the power performance of lithium-ion batteries.
3.5. Electrochemical characterization
With the aim to verify the latter point, we investigated the performance of LVP-based electrodes in the three investigated electrolytes. Specifically, we carried out a C-rate test, at 30 °C, using currents ranging from 1 C to 20 C. In order to have a more exhaustive comparison, also the conventional electrolyte 1 M LiPF6 in EC:DMC was used for this test. As shown in Fig. 5, when a current density corresponding to 1 C was applied, LVP cathodes displayed in both PIL-based electrolytes specific capacities in the order of 120 mA h g−1. These values are close to the theoretical capacity of LVP (131 mA h g−1 between 3.0 V and 4.3 V vs. Li/Li+) and they were comparable to those observed for the same electrodes in the conventional electrolyte. This capacity was 10% higher than that observed for 0.5 M LiTFSI in PYR14TFSI (110 mA h g−1). When the C-rate was increased, the differences between AIL- and PIL-based electrolytes became even more severe. At 5 C, the LVP electrodes used in combination with PIL-based electrolytes displayed 30% higher capacity than those cycled with the AIL-based electrolyte. At 20 C, the LVP electrode cycled with 0.5 M LiTFSI in PYR14TFSI delivered a capacity of ca. 20 mA h g−1, while the ones cycled with 0.5 M LiTFSI in PYRHHTFSI and 0.5 M LiTFSI in PYRH4TFSI displayed a capacity three times higher, in the order of 55–60 mA h g−1. As shown in the figure, at high C-rates (higher than 5 C) the performance of all IL-based electrolytes was still lower than the one obtained with the conventional electrolyte. Nevertheless, the results of this investigation indicate that the presence of loosely coordinated Li+ ions in PIL-based electrolytes, compared to that in AIL-based electrolytes, has dramatic consequences on the electrode performance.In order to understand the reasons of the different behavior of the LVP electrodes in the investigated electrolytes, impedance spectra were recorded at the fully lithiated state (after full discharge) and in a partially lithiated state (at the first plateau of the discharge). The latter state was selected in order to have indication about the charge-transfer resistance in the electrolytes. Since the two PILs display similar behavior, only the electrolyte 0.5 M LiTFSI in PYRH4TFSI was used for this test. As shown in Fig. 6, in all electrolytes the impedance spectra of the LVP electrodes in the fully lithiated state presented a semicircle at high-medium frequency, followed by a diffusion part at low frequency.26 As expected, the charge-transfer resistance observed in the conventional electrolyte was the lowest (Fig. 6b) which was also shown in the literature before.27 Both IL-based electrolytes show higher charge-transfer resistance due to their lower conductivity. Nevertheless, it is important to notice that the overall resistance observed in the PIL-based electrolytes (Fig. 6d) was lower than that observed in the AIL-based one (Fig. 6f). The impedance spectra of the LVP electrodes in the partially lithiated state were significantly different. In all electrolytes a second semicircle, located in the medium frequency region, appeared. The presence of this additional semicircle in the partially lithiated state is correlated with the charge-transfer resistance associated with the lithium insertion into the LVP structure.26,28 Also in this case, the charge-transfer resistance observed in the conventional electrolyte was the lowest. However, it is very interesting to notice the remarkable difference between the PIL-based electrolyte and the AIL-based one. As shown, the semicircle in the case of 0.5 M LiTFSI in PYR14TFSI was significantly larger than the one of 0.5 M LiTFSI in PYRH4TFSI, indicating the presence of a much higher charge transfer resistance in the AIL. Taking into account the Raman results reported above, it appears reasonable to suppose that the lithium desolvation process is easier in PILs than in AILs, and that this difference could be the origin of the different charge-transfer resistance between these two types of ILs. As shown in Fig. 5, this different charge-transfer resistance affects significantly the behavior of the electrodes during tests at high current densities and, most likely, is one of the main responsible reasons for the higher capacity observed in PIL-based electrolytes compared to the AIL-based one.
Finally, also the cycling stability of the LVP electrodes in the electrolytes 0.5 M LiTFSI in PYRHHTFSI and 0.5 M LiTFSI in PYRH4TFSI was investigated. The tests were carried out at 30 °C using a C-rate equal to 1 C. As shown in Fig. 7, the LVP electrodes display high capacity, between 120and 130 mA h g−1, in both PIL-based electrolytes. These values are in agreement with those observed during the C-rate test and are comparable with those shown by the LVP electrode in conventional electrolytes.16 As indicated in the figure, such high capacity can be fully maintained for 100 cycles and it is important to notice that the efficiency of the charge–discharge process was close to 100% during all cycles. These results clearly indicate that systems containing dry PIL-based electrolytes can also display high cycling stability.
 | ||
| Fig. 7 Cycling stability of LVP electrodes in the electrolytes 0.5 M LiTFSI in PYRHHTFSI and 0.5 M LiTFSI in PYRH4TFSI at 30 °C. The constant current cycling was carried out at a C-rate of 1 C. | ||
4. Conclusions
Protic ionic liquids display all typical and favourable properties of ILs, but they have the advantage of being easier to synthesize and cheaper compared to aprotic ionic liquids. In this work, we considered two pyrrolidinium-based PILs, PYRHHTFSI and PYRH4TFSI, in view of their use as electrolytes for LIBs. We showed that the (dry) electrolytes 0.5 M LiTFSI in PYRHHTFSI and 0.5 M LiTFSI in PYRH4TFSI display conductivity, viscosity and lithium-ion self-diffusion coefficient comparable to those of a pyrrolidinium-based AIL. However, they have the important advantage of displaying an improved performance of LVP-based electrodes during tests at a high C-rate. The lithium ions in PIL-based electrolytes do not move faster than in AIL-based electrolytes due to their self-diffusion coefficients. However, fewer TFSI− anions form the solvation sphere of Li+ in the investigated PILs. We showed that the improved performance of LVP electrodes in the PIL-based electrolytes is related to a reduced charge-transfer resistance at the LVP–electrolyte interface. Also an increased Li+ mobility in the presence of an electric field in the PILs compared to the one in the AIL (which is not reflected by the self-diffusion coefficients) could play a significant role and investigations about the latter point are currently in progress. Taking into account that the limited performance at a high rate of IL-based LIBs is presently considered as one of the main limitations of these devices, the use of PIL-based electrolytes can be regarded as a new and promising strategy to overcome this drawback. Additionally, since PILs are typically cheaper than AILs, the introduction of this innovative electrolyte could also be of importance for the development of safe and cheaper IL-based lithium-ion batteries.Acknowledgements
The authors wish to thank the Westfälische Wilhelms-Universität Münster, the Ministerium für Innovation, and Wissenschaft und Forschung des Landes Nordrhein-Westfalen (MIWFT) for the financial support.Notes and references
- B. Scrosati and J. Garche, J. Power Sources, 2010, 195, 2419–2430 CrossRef CAS PubMed.
- M. Armand, F. Endres, D. R. MacFarlane, H. Ohno and B. Scrosati, Nat. Mater., 2009, 8, 621–629 CrossRef CAS PubMed.
- A. Balducci, S. S. Jeong, G. T. Kim, S. Passerini, M. Winter, M. Schmuck, G. B. Appetecchi, R. Marcilla, D. Mecerreyes, V. Barsukov, V. Khomenko, I. Cantero, M. I. De, M. Holzapfel and N. Tran, J. Power Sources, 2011, 196, 9719–9730 CrossRef CAS PubMed.
- J. F. Wishart, Energy Environ. Sci., 2009, 2, 956–961 CAS.
- A. Guerfi, M. Dontigny, P. Charest, M. Petitclerc, M. Lagace, A. Vijh and K. Zaghib, J. Power Sources, 2010, 195, 845–852 CrossRef CAS PubMed.
- R.-S. Kühnel, N. Böckenfeld, S. Passerini, M. Winter and A. Balducci, Electrochim. Acta, 2011, 56, 4092–4099 CrossRef PubMed.
- M. Anouti, M. Caillon-Caravanier, Y. Dridi, H. Galiano and D. Lemordant, J. Phys. Chem. B, 2008, 112, 13335–13343 CrossRef CAS PubMed.
- H. Matsuoka, H. Nakamoto, M. A. B. H. Susan and M. Watanabe, Electrochim. Acta, 2005, 50, 4015–4021 CrossRef CAS PubMed.
- M. Anouti, E. Couadou, L. Timperman and H. Galiano, Electrochim. Acta, 2012, 64, 110–117 CrossRef CAS PubMed.
- A. Brandt, J. Pires, M. Anouti and A. Balducci, Electrochim. Acta, 2013, 108, 226–231 CrossRef CAS PubMed.
- T. Yasuda and M. Watanabe, MRS Bull., 2013, 38, 560–566 CrossRef CAS.
- N. Böckenfeld, M. Willeke, J. Pires, M. Anouti and A. Balducci, J. Electrochem. Soc., 2013, 160, A559–A563 CrossRef PubMed.
- S. Menne, J. Pires, M. Anouti and A. Balducci, Electrochem. Commun., 2013, 31, 39–41 CrossRef CAS PubMed.
- L. Timperman, P. Skowron, A. Boisset, H. Galiano, D. Lemordant, E. Frackowiak, F. Beguin and M. Anouti, Phys. Chem. Chem. Phys., 2012, 14, 8199–8207 RSC.
- P. Stilbs, Prog. Nucl. Magn. Reson. Spectrosc., 1987, 19, 1–45 CrossRef CAS.
- N. Böckenfeld and A. Balducci, J. Power Sources, 2013, 235, 265–273 CrossRef PubMed.
- L. Timperman, H. Galiano, D. Lemordant and M. Anouti, Electrochem. Commun., 2011, 13, 1112–1115 CrossRef CAS PubMed.
- F. Castiglione, E. Ragg, A. Mele, G. B. Appetecchi, M. Montanino and S. Passerini, J. Phys. Chem. Lett., 2011, 2, 153–157 CrossRef CAS.
- S. Duluard, J. Grondin, J.-L. Bruneel, I. Pianet, A. Grélard, G. Campet, M.-H. Delville and J.-C. Lassègues, J. Raman Spectrosc., 2008, 39, 627–632 CrossRef CAS.
- D. Brouillette, D. E. Irish, N. J. Taylor, G. Perron, M. Odziemkowski and J. E. Desnoyers, Phys. Chem. Chem. Phys., 2002, 4, 6063–6071 RSC.
- M. Herstedt, M. Smirnov, P. Johansson, M. Chami, J. Grondin, L. Servant and J. C. Lassègues, J. Raman Spectrosc., 2005, 36, 762–770 CrossRef CAS.
- A. Martinelli, A. Matic, P. Johansson, P. Jacobsson, L. Börjesson, A. Fernicola, S. Panero, B. Scrosati and H. Ohno, J. Raman Spectrosc., 2011, 42, 522–528 CrossRef CAS.
- M. Castriota, T. Caruso, R. G. Agostino, E. Cazzanelli, W. A. Henderson and S. Passerini, J. Phys. Chem. A, 2005, 109, 92–96 CrossRef CAS PubMed.
- J.-C. Lassegues, J. Grondin and D. Talaga, Phys. Chem. Chem. Phys., 2006, 8, 5629–5632 RSC.
- S. Menne, T. Vogl and A. Balducci, Phys. Chem. Chem. Phys., 2014, 16, 5485–5489 RSC.
- X. H. Rui, N. Ding, J. Liu, C. Li and C. H. Chen, Electrochim. Acta, 2010, 55, 2384–2390 CrossRef CAS PubMed.
- L. S. Plashnitsa, E. Kobayashi, Y. Noguchi, S. Okada and J.-i. Yamaki, J. Electrochem. Soc., 2010, 157, A536–A543 CrossRef CAS PubMed.
- J.-M. Atebamba, J. Moskon, S. Pejovnik and M. Gaberscek, J. Electrochem. Soc., 2010, 157, A1218–A1228 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |



