Environmental SEM monitoring of Ce1−xLnxO2−x/2 mixed-oxide microstructural evolution during dissolution
Abstract
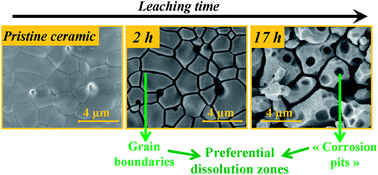
The microstructural evolution of several Ce1−xLnxO2−x/2 (Ln = Nd, Er, x = 0.28; 0.59 and 0.76) sintered pellets was studied during their dissolution in 4 M HNO3 at 90 °C or 60 °C. Environmental Scanning Electron Microscopy (ESEM) experiments were developed to follow the changes in the sample microstructure and their consequences on the dissolution evolution by monitoring a constant zone of the material throughout the alteration process. The LnIII content was the parameter affecting most of the dissolution kinetics of such solid solutions. From a microstructural point of view, grain boundaries dissolve preferentially during the first dissolution step due to their relative weakness compared to the well-crystallized bulk material. Crystal defects, namely screw and edge dislocations, constitute the second kind of preferential dissolution zone and induce the formation and growth of corrosion pits, mainly of pyramidal shape on the whole surface. Both microstructural parameters are responsible for significant and non-linear increase of the reactive surface developed at the solid/liquid interface and thus important modifications of the topology. On sintered samples and even more clearly on the powdered Ce0.72Nd0.28O1.86 sample, the formation of a gelatinous layer resulting from processes occurring in the back-end of the dissolution process (e.g. local saturation of the leaching solution), was evidenced through ESEM observations. This gel is also presumably responsible for the progressive decrease of the normalized dissolution rate. From these observations, it appears that the study of material dissolution kinetics can clearly benefit from the monitoring of the microstructure evolution and particularly that of the reactive surface area during dissolution.
- This article is part of the themed collection: 2014 Journal of Materials Chemistry A Hot Articles

 Please wait while we load your content...
Please wait while we load your content...