Interfacial dynamics in foams and emulsions
Anniina
Salonen
,
Wiebke
Drenckhan
and
Emmanuelle
Rio
Laboratoire de Physique des Solides, Bâtiment 510, Université Paris Sud, 91405 Orsay cedex, France
Due to the high energetic cost of interfaces, they obey strict laws of area minimisation, which controls how bubbles and drops pack together, providing them with well-defined and elegant shapes and topologies. For the same reason, the closely packed bubbles and drops confer complex mechanical properties on foams and emulsions, turning them into visco-elastic-plastic yield-stress fluids. The flow and yielding of concentrated emulsions under shear are discussed in the contributions of Knowlton et al. (DOI: 10.1039/c4sm00531g) and Mansard et al. (DOI: 10.1039/c4sm00230j). Tight packings of bubbles and drops call upon another important branch of physics and physical chemistry: that of the stability of the thin liquid films that separate them. These may break and lead to coalescence or allow the passage of the dispersed phase (coarsening or Ostwald ripening). An elegant method to induce coalescence has been studied by Mensire et al. (DOI: 10.1039/c4sm00476k), where they inject pure water into a dry foam to locally dilute the surfactants and induce coalescence. Most stabilising agents can only slow down the related ageing of the dispersion, which is the fate of most kinetically – though not thermodynamically – stable systems.
Hence, in order to fully understand and control the behaviour of foams and emulsion, one needs to understand their properties at all the relevant length-scales, ranging from the nanometric to the macroscopic (see Fig. 1). Looking at foams or emulsions on the macroscopic scale, they are highly turbid, with mechanical properties ranging from slightly viscous at high continuous phase fractions to highly viscoelastic at low continuous phase fractions (such that the bubbles or droplets are in close contact and deformed). Zooming into the material, one starts to distinguish individual bubbles or droplets, the size and polydispersity of which is an important parameter influencing the stability and mechanical properties. As one looks closer still, in foams and in concentrated emulsions, we have another length-scale linked to the size of the interstices between the droplets or bubbles. At the smallest length-scales (down to a few nanometers) we have the interfaces between the fluids, where the stabilisers are found in the form of surfactants, proteins, polymers or particles (or mixtures).
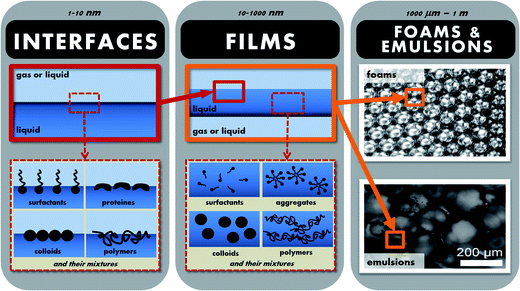 | ||
| Fig. 1 Illustration of the different length-scales of a foam or an emulsion ranging from the nanometric to the macroscopic. | ||
The macroscopic properties of foams and emulsions are the result of intricate interplay between the detailed structure and physical phenomena at all these different length-scales. For example, their rheology and ageing depend not only on the size and density of bubbles or droplets, but also on the mechanical properties of the interfaces or the fluid that surrounds them.
This hierarchy of length-scales, all with their own dynamics and structure, has led to the development of a wide range of model experiments to explore ageing and rheology of foams and emulsions. For example, experiments are carried out on individual interfaces (monolayers), individual films, on one or two bubbles or drops, or a single layer of bubbles (2D foams) or drops. Such model experiments and their theoretical description have led to important advances in our understanding of foam and emulsion destabilisation and flow. Such model experiments have been used to discuss the link between surface rheology and drainage of curved surfaces in Saad Bhamia et al. (DOI: 10.1039/c3sm52934g), while Scheid et al. (DOI: 10.1039/c4sm00718b) show the importance of gas dissolution in antibubble dynamics. Such experiments are also invaluable tools for understanding how the molecular structure of the stabilisers is linked to the foam properties. These links are discussed in Saulnier et al. (DOI: 10.1039/c4sm00326h) for the case of surfactants and surfactant mixtures, while von Klitzing and Hauser (DOI: 10.1039/c4sm01241k) review the stability of polymer films.
Another way to help disentangle the existing issues in foam and emulsion stability or rheology is to create monodisperse samples. The development of micro- and millifluidic tools has enabled the generation of samples where all the droplets or bubbles have the same size and may self-order into periodic structures under the influence of gravity or confinement, thus giving rise to reproducible dispersions with well-controlled properties. The generation and dynamics of bubbles in microfluidic devices is reviewed by Huerre et al. (DOI: 10.1039/c4sm00595c).
Another way to reduce some of the complexity of foam and emulsion science is to attempt to switch off gravity-driven drainage. Many European teams have been working over the last 15 years in order to prepare microgravity experiments on the international space station. Insights into the drainage of metal foams in varying degrees of gravity are shown by Garcia-Moreno et al. (DOI: 10.1039/c4sm00467a).
Particular attention has been paid in recent years to the mechanical properties of fluid interfaces. This is not only motivated by the role of interfaces in foam and emulsion science, but also because fluid interfaces are ideal as models for two-dimensional systems. In recent years the use of microrheological tools has become more common, and comparisons with macrorheological measurements have not always been conclusive. Samaniuk and Vermant (DOI: 10.1039/c4sm00646a) discuss the origins of these discrepancies and show that in many cases the two methods can be brought into agreement as suitable precautions are taken. The adsorption and desorption dynamics and even equilibrium behaviour of interfacially active agents is still attracting interest as, particularly for ionic surfactants, their behaviour cannot be fully explained (see review by Fainerman et al. (DOI: 10.1039/c4sm00463a) for example). Many open questions also remain in the description of interfaces containing solid and slightly softer (gels, proteins,…) particles, mixing mechanical and thermodynamic descriptions in many ways. The interest in such systems is reflected by the number of articles in this issue describing the mechanical properties of interfacially active proteins (Allan et al. (DOI: 10.1039/c4sm00484a) and Golemanov et al. (DOI: 10.1039/c4sm00406j)) or different types of particles (Stocco et al. (DOI: 10.1039/c4sm00482e), Pinaud et al. (DOI: 10.1039/c4sm00562g), Deshmukh et al. (DOI: 10.1039/c4sm00566j), Maestro et al. (DOI: 10.1039/c4sm00047a)). Having been used for quite some time to create extremely stable Pickering emulsions, their systematic use in the generation of highly stable foams is much more recent. In this issue Deleurence et al. (DOI: 10.1039/c4sm00237g) show how latex particles can be used for the stabilisation of foams. In order to further extend the uses of Pickering emulsions, biocompatible and food grade particles are required. An example of such a system is presented by Destribats et al. (DOI: 10.1039/c4sm00179f), where emulsions are stabilised using whey protein microgel particles.
Particles are not only found at the interfaces, but in many industrial products solid particles are an integral part of the continuous phase. Rouyer et al. (DOI: 10.1039/c4sm00496e) show how easily foams can be clogged by hydrophilic particles as they are trapped in the nodes of the foam. Mixtures of oil droplets and air bubbles in the form of foamed emulsions are also very common. The added oil droplets can increase the stability of the foams or lead to their faster destabilisation. Piroird et al. (DOI: 10.1039/c4sm00538d) show how an added oil droplet spreads inside the foam network and how it can lead to the rupture of the foam films.
Important advances have been achieved in foam and emulsion science thanks to an increasingly close collaboration between mathematicians, physicists, physical chemists and chemists. Bringing people from different disciplines together in a fruitful manner – especially in the early stages of the development of a field – requires the patient work of some charismatic scientists with a wide scientific background who are able to recognise and begin building the bridges that need to be created between different perspectives and cultures. Dominique Langevin is one of the scientists who has played a major role in this process. As such, the breadth of articles in this issue dedicated to her retirement reflects not only the complexity of foam and emulsion science, but also the range of subjects to which Dominique Langevin has contributed during her successful career.
The different articles in this issue – and the articles they cite – show that foam, emulsion and interface science has advanced quite significantly over the past few decades. Many of the key phenomena, like drainage, coarsening or rheology have found a reasonably solid description. Some of the key questions that remain to be tackled are concerned with the attempt to definitively link the physical properties of the stabilising agent to the foam/emulsion stability in an attempt to predict in a reliable manner the foaming and emulsification properties of a substance. Part of this work is motivated by an increasing concern about environmental issues, since many of the most commonly used stabilisers are derived from petrol. Important investigations into foam and emulsion stability extend to dispersions made from non-aqueous fluids and complex fluids, such as liquid crystals, polymer solutions/melts (Egger et al. (DOI: 10.1039/c4sm00494a)), particle dispersions or emulsions. In a growing number of cases these complex foams or emulsions serve as precursors for the generation of solid porous materials with well-controlled structural properties. A second important field needs to establish an understanding of the coupling of different phenomena – such as drainage, rheology and stability. Last but not least, new fields are being opened at the moment. The most prominent examples include the use of emulsions as model “active particles”, as presented by Herminghaus et al. (DOI: 10.1039/c4sm00550c) or “smart foams and emulsions”, which may be stimulated using external stimuli such as temperature gradients, light, electric or magnetic fields. The design criteria for polymers suitable for the creation of such smart emulsions are discussed by Besnard et al. (DOI: 10.1039/c4sm00596a).
| This journal is © The Royal Society of Chemistry 2014 |



