Structure of bovine β-lactoglobulin–lactoferrin coacervates†
Abstract
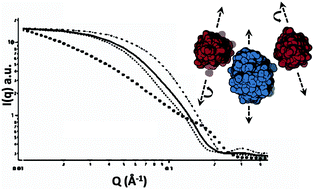
Lactoferrin (LF) and β-lactoglobulin (BLG) are among the protein pairs that exhibit heteroprotein coacervation, a unique and relatively unexamined type of liquid–liquid phase separation (LLPS). In prior work we found that LF and BLG undergo coacervation at highly constrained conditions of pH, ionic strength and protein stoichiometry. The molar stoichiometry in coacervate and supernatant is LF : BLG2 1 : 2 (where BLG2 represents the 38 kDa BLG dimer), suggesting that this is the primary unit of the coacervate. The precise balance of repulsive and attractive forces among these units, thought to stabilize the coacervate, is achieved only at limited conditions of pH and I. Our purpose here is to define the process by which such structural units form, and to elucidate the forces among them that lead to the long-range order found in equilibrium coacervates. We use confocal laser scanning microscopy (CLSM), small angle neutron scattering (SANS), and rheology to (1) define the uniformity of interprotein spacing within the coacervate phase, (2) verify structural unit dimensions and spacing, and (3) rationalize bulk fluid properties in terms of inter-unit forces. Electrostatic modeling is used in concert with SANS to develop a molecular model for the primary unit of the coacervate that accounts for bulk viscoelastic properties. Modeling suggests that the charge anisotropies of the two proteins stabilize the dipole-like LF(BLG2)2 primary unit, while assembly of these dipoles into higher order equilibrium structures governs the macroscopic properties of the coacervate.

 Please wait while we load your content...
Please wait while we load your content...