 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Emerging bioinspired polymers: glycopolypeptides
Kai-Steffen
Krannig
and
Helmut
Schlaad
*
Max Planck Institute of Colloids and Interfaces, Department of Colloid Chemistry, Research Campus Golm, 14424 Potsdam, Germany. E-mail: Helmut.Schlaad@mpikg.mpg.de
First published on 27th March 2014
Abstract
This article highlights the very recent advances in glycopolypeptide synthesis via NCA polymerization and first studies on stimuli-responsive solution behavior and self-assembling structures. Yet glycopolypeptides are almost exclusively considered as smart biofunctional materials for use in biomedical applications, for instance in targeted drug delivery, but also have high potential for usage as structural materials to fabricate bioinspired hierarchical structures.
1. Introduction
Bioinspired or biohybrid polymers are an emerging class of materials that are designed to combine advantageous features of synthetic polymers, such as solubility, processability, and scalability, with those of biological entities like chirality, selective recognition, or signaling.1,2 Recent efforts have been focused on sugar-containing polymers, including carbohydrate-synthetic copolymers, also block copolymers, and polymers with pendant carbohydrates – the latter being usually referred to as glycopolymers.3Since the first reported examples in the early 1960s,4–6 many different glycopolymers have been produced by free or controlled radical polymerization of vinyl monomers and ring-opening metathesis polymerization of norbornenes.7–9 All backbones are carbon chains, which have no other functions than to connect the carbohydrate residues and to serve as spacer units. Polypeptides, on the other hand, have the intriguing ability to fold into secondary, tertiary, and higher order structures due to multiple non-covalent interaction amino acid units and are inherently biocompatible and biodegradable. Naturally occurring glycosylated peptides and proteins display a wide range of biological functions including anti-freezing,10 mediation of recognition events, proliferation of cells, and inflammatory reactions.11–14 Hence their synthetic counterparts are also expected to have great potential as biomedical materials (e.g., drug carriers or scaffolds for tissue repair) and to serve as versatile tools for probing carbohydrate–protein interactions.15,16 Synthetic glycopeptides have been known for 20 years,17 nevertheless the production of well-defined glycopolypeptides in larger quantities is still a challenging task and the understanding of their hierarchical assembly, as an indispensable condition to a smart design of advanced functional materials, is in its infancy.
This article presents a selection of very recent advances in the field of glycopolypeptides, covering the synthesis, stimuli-responsive secondary structures and self-assemblies, and biological applications. Comprehensive and more detailed information can be found in a number of excellent reviews and perspectives that have been published in the last year.18–22
2. Synthesis of glycopolypeptides
Two different pathways are applied to produce glycopolypeptides (Fig. 1). The first route involves the synthesis of a glycosylated N-carboxyanhydride, referred to as glyco-NCA, which then is polymerized in a controlled manner to yield high molar mass glycopolypeptides. The main issue or problem is the synthesis and purification of glyco-NCA, which requires a high level of synthesis expertise. The second route is the synthesis of a functionalizable polypeptide precursor chain, which is subjected to post-polymerization modification to introduce the carbohydrate residues. The synthesis is modular and much less elaborate than the first route without compromising the degree of glycosylation, however, the glycopolypeptides obtained by modification of polypeptides have considerably lower molar masses than the ones produced by glyco-NCA polymerization.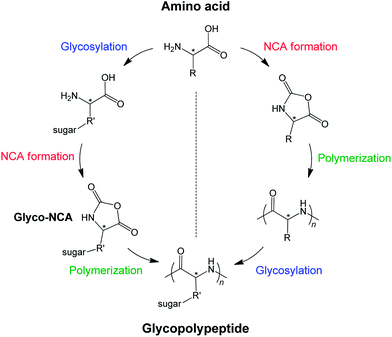 | ||
| Fig. 1 Synthetic pathways towards glycopolypeptides: (1) synthesis and polymerization of glyco-NCA (left) and (2) synthesis of a functionalizable polypeptide and subsequent glycosylation (right). | ||
2.1 Synthesis and polymerization of glyco-NCAs
Okada et al. described the first synthesis of a glycopeptide through amine-initiated ring-opening polymerization of glyco-NCA, namely O-(tetra-O-acetyl-β-D-glucopyranosyl)-L-serine.17 This glyco-NCA was obtained by coupling tetra-O-acetylated glucose to serine, as described by Rüde et al.,23 and subsequent reaction of the amino acid with phosgene. Application of primary amine initiators produced well-defined glycopeptides with number-average molar masses (Mn) of about 10 kg mol−1 and low dispersity, Mw/Mn ≈ 1.1 (ratio of weight- over number-average molar mass). The living character of the polymerization was demonstrated by the addition of a second monomer, alanine–NCA, to yield block copolypeptides.17The synthesis of higher molar mass glycopolypeptides requires glyco-NCAs in high purity, which is made difficult by their high reactivity. Recently, in 2010, Deming et al. succeeded in the chromatographic purification of glycosylated L-lysine-NCAs and were able to achieve high molar mass polypeptides (Mn ≈ 160 kg mol−1) with low dispersity.24,25 Peracetylated sugars (glucose, galactose, or mannose) were coupled to L-lysine in a four-step procedure and the amino acid transferred into NCA by treatment with dichloro(methoxy)methane. The glyco-NCAs were afterwards purified by anhydrous flash chromatography. Polymerization using a transition metal catalyst, (PMe3)4Co, yielded homo and block copolypeptides, which after deacetylation could be readily dispersed in water.
Sen Gupta et al. reported another procedure towards glyco-NCAs based on L-lysine.26–28 A protected lysine, CbzLys(Boc)OBn (Cbz = benzyloxycarbonyl, Boc = tert-butyloxycarbonyl, Bn = benzyl), was coupled with the 1,2-orthoesters of either glucose or mannose in the presence of a gold catalyst. After removal of the acid protecting group by hydrogenation, the amino acid was converted into the NCA by the Fuchs–Farthing method using triphosgene (and α-pinene as hydrogen chloride scavenger). These lysine derived glyco-NCAs were purified by conventional recrystallization. The polymerization reactions of glyco-NCAs, initiated by 1-hexylamine, were only successful in the presence of the 1,8-bis(dimethylamino)naphthalene “proton sponge” neutralizing residual acid impurities (however, the exact role of the base is not fully understood yet). The obtained homo- and block copolypeptides exhibited low dispersity but usually much higher than expected molar masses.
Wenz et al. synthesized a sugar-appended lysine–NCA in a three-step procedure by glycosylation of 2-(2-isothiocyanatoethoxy)ethanol with 1-trichloroacetimidate-2,3,4,6-tetra-O-acetyl-glycopyranose and subsequent coupling of the isothiocyanate group to the ε-amino group of Cbz–lysine.29 Treatment with dichloromethyl(methoxy)methane yielded NCA, which was then copolymerized with PEGylated lysine- and ε-TFA–lysine–NCA using either triethylamine or the (bipy)Ni(COD) catalyst.
Glyco-NCAs were also obtained from L-cysteine by photochemical addition of the amino acid thiol to 1-allyl-2,3,4,6-tetra-O-acetyl-α-D-glucose or -galactose (employing 2,2-dimethoxy-2-phenylacetophenone as a photoinitiator and UV at 365 nm) and subsequent reaction with dichloro(methoxy)methane.30 Purification was done by either column chromatography or aqueous workup and repeated precipitation. The glyco-NCAs could be polymerized efficiently with the (PMe3)4Co initiator in tetrahydrofuran at room temperature to yield protected glycopolypeptides with molar masses (Mn) up to 90 kg mol−1 and low dispersity (<1.1).
The syntheses of other O- and S-linked glyco-NCAs based on L-threonine, L-serine, and L-cysteine have also been described.31 The acetobromo derivatives of glucose, galactose, or lactose were coupled to the respective amino acids in the presence of iodine as a Lewis acid promoter and subsequently reacted to the NCA using triphosgene and α-pinene in ethyl acetate. Polymerization reactions of such NCAs have not been reported yet.
Although a variety of glycosylated amino acid NCAs have been successfully prepared applying sophisticated organic chemistry protocols, the tedious multi-step synthesis and purification procedures and often low yields are severe drawbacks of this approach.
2.2 Post-polymerization glycosylation of synthetic polypeptides
Several approaches for the modification of already existing polypeptides have been described since 2010, attributable to the popularity of “click” chemistry.32–34 In particular, the copper-catalyzed azide–alkyne [3 + 2] cycloaddition (CuAAC) became a broadly applied technique in not only the synthesis of glycosylated monomers but also in the post-polymerization functionalization of polymers.7,35Zhang et al. synthesized a γ-chloropropyl-L-glutamate–NCA by the monoesterification of glutamic acid with 3-chloropropyl alcohol in the presence of chlorotrimethylsilane and subsequent treatment with triphosgene, and polymerized it using a hexamethyldisilazane initiator.36,37 The obtained polypeptides (Mn = 5–28 kg mol−1, dispersity ≈ 1.2) were reacted with sodium azide to give poly(γ-azidopropyl-L-glutamate), which allowed for quantitative addition of 1-propargyl-α-D-mannose via CuAAC. Alternatively, poly(γ-propargyl-L-glutamate) was prepared by primary amine initiated ring-opening polymerization of the corresponding amino acid–NCA and then reacted with sugar-azides applying CuAAC.38
The CuAAC approach was also applied to polypropargylglycine, avoiding any hydrolytically unstable ester linkage in the polypeptide side chains.39–41 Polypropargylglycine homopolymers and statistical and block copolymers (with γ-benzyl-L-glutamate) were synthesized by primary amine initiated ring-opening polymerization of propargylglycine–NCA (readily obtained by the reaction of the commercial amino acid with triphosgene) and were glycosylated with 1-azido-β-D-galactose in the presence of triethylamine and catalytic amounts of (PPh3)3CuBr in DMSO solution.
Polyallylglycines (and the related polydepsipeptides) have been introduced as another clickable polypeptide by thiol–ene chemistry, connecting functional groups to the polypeptide chains with a thioether linker (as in methionine).42,43 Polyallylglycines, as well as polypropargylglycines, are poorly soluble in common organic solvents or water, making the synthesis of higher molar mass polypeptides and quantitative modification difficult or even impossible. Polyallylglycine with rather a low molar mass of Mn < 2 kg mol−1 could only be modified quantitatively with 1-thio-β-D-glucopyranose, to yield poly[(3-(β-D-glucopyranosyl)thio)propylglycine], when the reaction was run in trifluoroacetic acid (TFA) over two days. This is not ideal because TFA is a hazardous solvent and may not be applicable to more sensitive biological substrates. Interestingly, low molar mass poly(N-allyl glycine) (Mn < 2 kg mol−1), which is the peptoid structural isomer to polyallylglycine, is soluble in water and could be readily converted into a glycopolypeptoid by photoaddition of 1-thio-β-D-glucopyranose within less than a day.44
Recently, statistical copolymers of allyl- or propargylglycine and γ-benzyl-L-glutamate, exhibiting much better solubility, have been synthesized and glycosylated in organic media or in aqueous media (acetate buffer) after debenzylation of the glutamate units.45,46 The single addition of the thio-sugar to allylglycine units was found to proceed smoothly and quantitatively, but double addition of sugar to propargylglycine proved to be more difficult (only achieving <1.5 equivalents). Importantly, the modification reaction occurs in water using unprotected sugars and may well be applied to other thiol bearing biological materials.
Functional cationic polypeptides including glycopolypeptides have been synthesized by the quantitative alkylation of poly(L-methionine)s with alkyl halides or triflates.47 For instance, methionine alkylation with 2-(2,3,4,6-tetra-O-acetyl-α-D-galactopyranosyl)ethyl triflate in a solvent mixture of dichloromethane–acetonitrile at 20 °C produced the corresponding S-linked glycopolypeptide in 97% yield. Interestingly, alkylation of the methionine units is reversible and dealkylation can be induced when desired.48
N-Linked glycopolypeptides were obtained by condensation of β-glycosylamines to poly(L-glutamic acid)49 or by aqueous amide coupling of D-glucosamine hydrochloride to poly(L-glutamic acid) (Mn = 9–180 kg mol−1) using 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride (DMT-MM) as a coupling agent.50 The latter reaction was performed without any organic solvents, additives, or buffers, and the degree of substitution could be controlled by the molar ratio of DMT-MM to glutamate to about 80%. A similar approach has been used for glycosylation of a protein sequence containing glutamic acid residues with β-D-galactosamine using N,N,N′,N′-tetramethyl-O-(1H-benzotriazol-1-yl)uranium hexafluorophosphate (HBTU) as a coupling agent under basic conditions.51 Alternatively, poly(L-lysine) was glycosylated with D-gluconolactone in the presence of diisopropylethylamine in DMSO–water at room temperature.52
The post-polymerization glycosylation approach appears to be a good alternative to glyco-NCA polymerization, though there are still limitations, applying to some but not all strategies, regarding molar mass and degree of glycosylation. However, the polypeptide precursors are modular platform materials that can be decorated with multiple substrates to yield heterofunctional glycopolypeptides for a variety of tasks.
3. Aqueous solution and self-assembly behavior
A key feature of polypeptides is their intrinsic ability to form distinct secondary and higher-order structures, which is usually not observed for conventional synthetic polymers (e.g., polyolefines, polyacrylates, or polyesters). Exceptions being materials such as polyamides (nylons) which form sheet-like superstructures through intermolecular hydrogen bonding, ultimately leading to properties such as high toughness. Synthetic polypeptide structures can reach a higher complexity and sophistication and, in addition, adapt to changes in environmental conditions (temperature, solution pH, ionic strength, etc.) or respond to other kinds of chemical, physical, or biological stimuli, making them promising building blocks for bioinspired or biomimetic structure formation.3.1 Stimuli-responsive behavior
Similar thermo-responsive behavior could also be observed for poly[L-glutamate-stat-(3-(β-D-glucopyranosyl)thio)propylglycine] (Mn ≈ 15 kg mol−1).54 Absolute helicities were determined to be 60–70% (maximum value for pH 3.5) at 37 °C, roughly only 10% less to room temperature. The helicity further decreases down to about 40% upon increasing the temperature to 90 °C. The original secondary structure (helicity) could be fully retained after cooling back to room temperature, indicating that the conformational transition (or denaturation) is a fully reversible process.
Both glycopolypeptides undergo temperature-induced helix–coil transition, though the observed changes in helicity are rather minor in the relevant temperature window of biomedical applications.
Poly[L-glutamate-stat-(N-glucopyranosyl)-L-glutamine]s adopt random coil conformation at neutral pH and helical conformation at acidic pH 4.2 (in 0.01 M acetate buffer).50 The helicity was found to decrease with increasing fraction of glycosylated glutamine units, and the helicity of a polypeptide chain with 78% glutamine units was just about 10%. Only the copolypeptides with 63% or less glycosylated units were able to undergo a pH-responsive and reversible helix-to-coil transition.
Poly[L-glutamate-stat-(3-(β-D-glucopyranosyl)thio)propylglycine]s exhibited similar pH-responsive behavior including reversible helix–coil transition without β-sheet formation.54 Helices remained stable and soluble in water or saline solution down to pH 3.5.45 The maximum helicity was about 78% at pH 4.0, irrespective of the composition of the polypeptide chain, and was even higher for a poly(L-glutamate) homopolymer (71%) of the same chain length.
Poly[S-(1-propyl-α-D-galactopyranosyl)-L-cysteine] adopts an α-helical conformation in aqueous solution, which is destabilized and transformed into a random coil after oxidation of the thioether to sulfone (reduction pathway not reported yet).30 Mild oxidation of the linker to the sulfoxide, on the other hand, did not cause destabilization of the α-helix. It is thought that the increased polarity and interaction of the sulfone with water lead to disruption of hydrophobic packing of side chains and increased steric crowding around the polypeptide backbone, thereby destabilizing the α-helix. Interestingly, glycosylated poly(L-homocysteine)s, which carry an additional methylene group between the backbone and sulfur, adopt helical conformations irrespective of the oxidation state. Poly[L-(3-(β-D-glucopyranosyl)thio)propylglycine] with a C3 spacer, however, prefers a disordered random coil conformation.54 Apparently, the spacing between sulfur and the backbone is a sensitive balance with a tremendous impact on the chain folding.
Poly[L-glutamate-stat-(3-(β-D-glucopyranosyl)thio)propylglycine] with a thioether linker in the side chain is also expected to respond to a redox stimulus (in addition to temperature and pH, see above), though there are no current reports.
3.2 Self-assemblies in aqueous solution
Polypeptide scaffolds can produce a large variety of complex structures, which is controlled by the chemistry and type of the side chain functional groups, i.e., primary structure. Sophisticated primary structures as in biological polypeptides or proteins have not yet been realized for synthetic polypeptides (via NCA polymerization), only simple codes like homopolymers or copolymers (statistical, alternating, gradient, or block structures; two or more comonomers). Polypeptides have been used in peptide–peptide or polymer–peptide (biohybrid) block copolymers to produce self-assemblies in water, particularly micelles, vesicles, and hydrogels, designated for use in biomedical applications.2 Until now, very few studies on the self-assembly behavior of synthetic glycopolypeptides have been reported.Lecommandoux, Heise et al. produced a variety of different morphologies of amphiphilic poly(γ-benzyl-L-glutamate)-block-(glyco-polypropargylglycine) copolypeptides by the nanoprecipitation technique (Fig. 2).40 Addition of water to a solution of the glycopolypeptide (hydrophilic weight fraction >50%) in dimethylsulfoxide (DMSO) and subsequent removal of the organic solvent by dialysis resulted in the formation of spherical and worm-like structures in water. Changing the order of addition of the solutions produced exclusively spherical structures such as micelles and small vesicles with an average diameter of <100 nm and a 5–10 nm thick membrane. All observed morphologies are non-equilibrium structures and resulted from kinetic trapping induced by the rigidity of the hydrophobic poly(γ-benzyl-L-glutamate) segment.
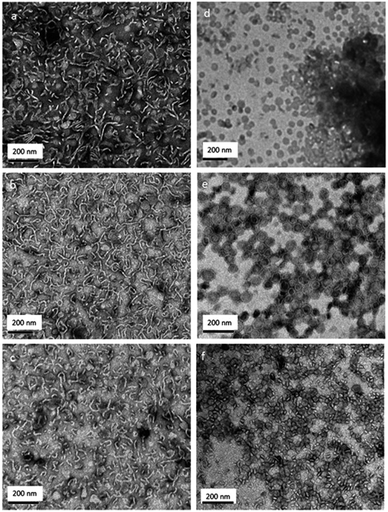 | ||
| Fig. 2 Transmission electron micrographs of poly(γ-benzyl-L-glutamate)-block-(glyco-polypropargylglycine) (PBLG-b-PGG) samples obtained by fast addition of (a–c) water in DMSO and (d–f) DMSO in water: (a and d) PBLG20-b-PGG18; (b and e) PBLG20-b-PGG25; (c and f) PBLG20-b-PGG32 (subscripts denote the average number of repeat units). Reprinted with permission from ref. 40, copyright (2012) American Chemical Society. | ||
Deming et al. described the aqueous self-assembly of two amphiphilic poly(L-leucine)-block-glyopolypeptide samples, where the hydrophobic segment had α-helical conformation (poly(L-leucine)) and the hydrophilic glycosylated segment adopted either α-helical (poly(α-D-galactopyranosyl-L-lysine)) or random coil conformation (poly(α-D-galactopyranosyl-L-cysteine sulfone)) (Fig. 3).59 The all-helical sample was found to assemble into micron sized irregular aggregates and plate-like objects, due to the rigidity of the hydrophilic domains. Poly(L-leucine)-block-poly(α-D-galactopyranosyl-L-cysteine sulfone) with a more flexible hydrophilic segment instead formed vesicles of about 100 nm in diameter (Fig. 3).
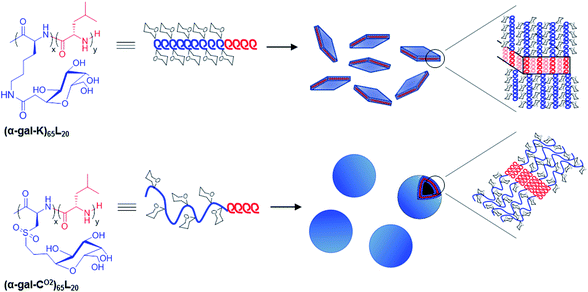 | ||
| Fig. 3 Structures of amphiphilic glycosylated diblock copolypeptides and observed self-assemblies in water. Reprinted with permission from ref. 59, copyright (2013) Royal Society of Chemistry. | ||
Sen Gupta described the self-assembly of amphiphilic glycopolypeptide–dendron block copolymers of glycosylated poly(L-lysine) attached to an aromatic dendron of generation 1 or 2 (see Fig. 4, top).60 Depending on the length of the glycopolypeptide (average number of repeat units: 14, 16, or 28) and dendron generation (1 or 2), either an organogel was obtained in DMSO or nanorods and micellar aggregates in aqueous solution (Fig. 4). The rod-like fibers, which exhibited a compartmentalized structure, were 50 nm in width and hundreds of nanometers in length, the length increases with decreasing mole fraction of the glycopolypeptide. The self-assembly behavior was explained by the cooperative effects of chain segregation and π–π-stacking of the dendrons. Interestingly, nanorods could only be observed for the L- but not for DL-glycopolypeptide–dendron copolymers, suggesting a correlation between the glycopolypeptide secondary structure and the self-assembly.
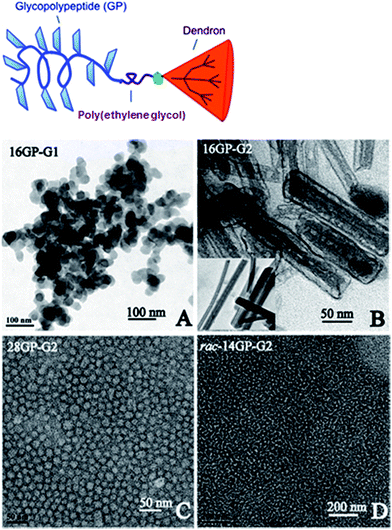 | ||
| Fig. 4 (Top) Schematic representation of glycopolypeptide–dendron (GPn–Gx) conjugates (n: average number of repeat units, x: dendron generation). (Bottom) Transmission electron micrographs of (A) GP16–G1, (B) GP16–G2, (C) GP28–G2, and (D) racGP14–G2 in water (negatively stained with uranyl acetate). Reprinted with permission from ref. 60, copyright (2012) American Chemical Society. | ||
Amphiphilic ABn heteroarm star (“tree-like”) copolypeptides based on poly(γ-benzyl-L-glutamate)-block-(oligosaccharide-polypropargylglycine) (A = poly(γ-benzyl-L-glutamate), B = dextran or hyaluronan, n = 5, average number of arms) were found to form very small spherical assemblies with sizes below 50 nm and low dispersity, by direct dissolution in water.41 Here, spherical particles with high curvature are formed due to the large hydrophilic weight fraction of >60%, despite the intrinsic rigidity of the hydrophobic poly(γ-benzyl-L-glutamate).
4. Biological applications
Lectin binding
Recognition is the first step in numerous biological processes based on cell–cell interactions. In many cases these recognition events are based on specific carbohydrate–protein (lectin) interactions that occur on the surface of cells.61 Lectins bind specifically but weakly to carbohydrates, and the binding can be enhanced by combining several carbohydrates in the same molecule (oligosaccharide or glycopolymer) or aggregate allowing for multiple binding events at the same time (cluster glycoside effect).7,35 However, the mechanisms and influence of the ligand structure on the multivalent binding interactions and the glyco code were largely unknown yet.Several studies have been reported to demonstrate that the synthetic glycopolypeptides can indeed recognize and specifically interact with lectins, usually by turbidity assays,27,28,38,40,45,49,59 surface plasmon resonance (SPR),62 and isothermal titration calorimetry (ITC).27 The lectins used were usually Concanavalin A (ConA) for the selective binding of glucose and mannose and Ricinus communis agglutinin (RCA120) for galactose. Interestingly, it has been found evidence that the degree of glycosylation (known as epitope density)15 affects the kinetics of lectin binding38 and that the secondary structure of the glycopolypeptide (α-helix or random coil) has no impact on the binding affinity.27
Wenz et al. showed that galactosylated fluorescent poly(L-lysine)s could be specifically incorporated in human T lymphocytes at 37 °C, hence are potentially useful for selective staining of cells for targeted drug delivery.29
Drug delivery
Just one study has been reported on the use of glycopolypeptide block copolymers, more on glycopeptide dendrimers,63,64 for targeted drug delivery.Feng et al. used the glycopolypeptide particles of glycosylated poly(L-lysine)-block-poly(tetrahydrofuran)-block-poly(L-lysine) for encapsulation and in vitro release of doxorubicin.52 The drug-loaded particles were spherical in shape and 100–150 nm in diameter. The doxorubicin release rate was found to be stable for about 10–15 h after an initial rapid release (about 50% within first 2 h) and was faster in an acidic than in a neutral environment. Additionally, the introduction of sugar improved the biocompatibility of the poly(L-lysine) segments.
5. Summary and outlook
The developments of new synthetic techniques and procedures toward glycopolypeptides through amino acid N-carboxyanhydride polymerization have enabled the preparation of a large number of new bioinspired functional polymer materials. Most of the synthesis studies and developments have been published after 2010, hence just a few reports exist yet on the advanced properties of glycopolypeptides, for instance stimuli-responsive solution behavior and self-assembly, or usage in biomedical applications.Glycopolypeptides, to our opinion, have immense potentials as bioinspired functional and structural materials, which have little been explored so far. Yet all research efforts are focused on multi-responsive glycopolypeptides and self-assemblies for advanced applications in biomedicine or life science, especially for targeted drug delivery and tissue engineering. Other usages of glycopolypeptides, like for instance fabrication of bulk materials and films with higher-order hierarchical structuring in a water-based process, have not been shown. Glycopolypeptides could be good candidates to mimic the natural hierarchical structures of collagens, α-keratins (based on helical glycopolypeptides), and β-keratins (based on β-sheet fibrous structures, which are unknown for glycopolypeptides yet) and open new avenues in materials science.
Acknowledgements
This work was supported by the Max Planck Society and the German Research Foundation within the IUPAC Transnational Pilot Call in Polymer Chemistry.References
- H. Schlaad and M. Antonietti, Eur. Phys. J. E, 2003, 10, 17–23 CrossRef CAS PubMed.
- H. G. Börner and H. Schlaad, Soft Matter, 2007, 3, 394–408 RSC.
- http://en.wikipedia.org/wiki/Glycopolymer .
- H. P. Panzer and R. L. Whistler, Chem. Eng. News, 1959, 37, 41 Search PubMed.
- T. P. Bird, W. A. P. Black, E. T. Dewar and D. Rutherford, Chem. Ind., 1960, 1331–1332 CAS.
- S. Kimura and M. Imoto, Makromol. Chem., 1961, 50, 155–160 CrossRef CAS.
- V. Ladmiral, E. Melia and D. M. Haddleton, Eur. Polym. J., 2004, 40, 431–449 CrossRef CAS PubMed.
- S. R. S. Ting, G. Chen and M. H. Stenzel, Polym. Chem., 2010, 1, 1392–1412 RSC.
- A. M. Eissa and N. R. Cameron, Adv. Polym. Sci., 2013, 253, 71–114 CrossRef CAS.
- Y. Yeh and R. E. Feeney, Chem. Rev., 1996, 96, 601–618 CrossRef CAS PubMed.
- C. R. Bertozzi and L. L. Kiessling, Science, 2001, 291, 2357–2364 CrossRef CAS.
- R. A. Dwek, Chem. Rev., 1996, 96, 683–720 CrossRef CAS PubMed.
- M. R. Pratt and C. R. Bertozzi, Chem. Soc. Rev., 2005, 34, 58–68 RSC.
- T. Buskas, S. Ingale and G.-J. Boons, Glycobiology, 2006, 16, 113R–136R CrossRef CAS PubMed.
- C. W. Cairo, J. E. Gestwicki, M. Kanai and L. L. Kiessling, J. Am. Chem. Soc., 2002, 124, 1615–1619 CrossRef CAS PubMed.
- J. E. Gestwicki, C. W. Cairo, L. E. Strong, K. A. Oetjen and L. L. Kiessling, J. Am. Chem. Soc., 2002, 124, 14922–14933 CrossRef CAS PubMed.
- K. Aoi, K. Tsutsumiuchi and M. Okada, Macromolecules, 1994, 27, 875–877 CrossRef CAS.
- J. Huang and A. Heise, Chem. Soc. Rev., 2013, 42, 7373–7390 RSC.
- C. Bonduelle and S. Lecommandoux, Biomacromolecules, 2013, 14, 2973–2983 CrossRef CAS PubMed.
- J. R. Kramer and T. J. Deming, Polym. Chem., 2014, 5, 671–682 RSC.
- M. A. Quadir, M. Martin and P. T. Hammond, Chem. Mater., 2014, 26, 461–476 CrossRef CAS.
- H. Lu, J. Wang, Z. Song, L. Yin, Y. Zhang, H. Tang, C. Tu, Y. Lin and J. Cheng, Chem. Commun., 2014, 50, 139–155 RSC.
- E. Rüde, O. Westphal, E. Hurwitz, S. Fuchs and M. Sela, Immunochemistry, 1966, 3, 137–151 CrossRef.
- J. R. Kramer and T. J. Deming, Biomacromolecules, 2010, 11, 3668–3672 CrossRef CAS PubMed.
- J. R. Kramer and T. J. Deming, J. Am. Chem. Soc., 2010, 132, 15068–15071 CrossRef CAS PubMed.
- D. Pati, A. Y. Shaikh, S. Hotha and S. S. Gupta, Polym. Chem., 2011, 2, 805–811 RSC.
- D. Pati, A. Y. Shaikh, S. Das, P. K. Nareddy, M. J. Swamy, S. Hotha and S. S. Gupta, Biomacromolecules, 2012, 13, 1287–1295 CrossRef CAS PubMed.
- S. Das, D. Pati, N. Tiwari, A. Nisal and S. S. Gupta, Biomacromolecules, 2012, 13, 3695–3702 CrossRef CAS PubMed.
- T. Stöhr, A. R. Blaudszun, U. Steinfeld and G. Wenz, Polym. Chem., 2011, 2, 2239–2248 RSC.
- J. R. Kramer and T. J. Deming, J. Am. Chem. Soc., 2012, 134, 4112–4115 CrossRef CAS PubMed.
- M. I. Gibson, G. J. Hunt and N. R. Cameron, Org. Biomol. Chem., 2007, 5, 2756–2757 CAS.
- H. C. Kolb, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2001, 40, 2004–2021 CrossRef CAS.
- J.-F. Lutz and H. Schlaad, Polymer, 2008, 49, 817–824 CrossRef CAS PubMed.
- C. R. Becer, R. Hoogenboom and U. S. Schubert, Angew. Chem., Int. Ed., 2009, 48, 4900–4908 CrossRef CAS PubMed.
- C. R. Becer, Macromol. Rapid Commun., 2012, 33, 742–752 CrossRef CAS PubMed.
- H. Tang and D. Zhang, Biomacromolecules, 2010, 11, 1585–1592 CrossRef CAS PubMed.
- H. Tang and D. Zhang, Polym. Chem., 2011, 2, 1542–1551 RSC.
- C. Xiao, C. Zhao, P. He, Z. Tang, X. Chen and X. Jing, Macromol. Rapid Commun., 2010, 31, 991–997 CrossRef CAS PubMed.
- J. Huang, G. Habraken, F. Audouin and A. Heise, Macromolecules, 2010, 43, 6050–6057 CrossRef CAS.
- J. Huang, C. Bonduelle, J. Thévenot, S. Lecommandoux and A. Heise, J. Am. Chem. Soc., 2012, 134, 119–122 CrossRef CAS PubMed.
- C. Bonduelle, J. Huang, E. Ibarboure, A. Heise and S. Lecommandoux, Chem. Commun., 2012, 48, 8353–8355 RSC.
- J. Sun and H. Schlaad, Macromolecules, 2010, 43, 4445–4448 CrossRef CAS.
- N. Franz and H.-A. Klok, Macromol. Chem. Phys., 2010, 211, 809–820 CrossRef CAS.
- J. W. Robinson and H. Schlaad, Chem. Commun., 2012, 48, 7835–7837 RSC.
- K.-S. Krannig and H. Schlaad, J. Am. Chem. Soc., 2012, 134, 18542–18545 CrossRef CAS PubMed.
- K.-S. Krannig, J. Huang, A. Heise and H. Schlaad, Polym. Chem., 2013, 4, 3981–3986 RSC.
- J. R. Kramer and T. J. Deming, Biomacromolecules, 2012, 13, 1719–1723 CrossRef CAS PubMed.
- J. R. Kramer and T. J. Deming, Chem. Commun., 2013, 49, 5144–5146 RSC.
- X. X. Zeng, T. Murata, H. Kawagishi, T. Usui and K. Kobayashi, Biosci., Biotechnol., Biochem., 1998, 62, 1171–1178 CrossRef CAS.
- R. Mildner and H. Menzel, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 3925–3931 CrossRef CAS.
- Y. Wang and K. L. Kiick, J. Am. Chem. Soc., 2005, 127, 16392–16393 CrossRef CAS PubMed.
- Z. Tian, M. Wang, A. Y. Zhang and Z. G. Feng, Polymer, 2008, 49, 446–454 CrossRef CAS PubMed.
- N. Lupu-Lotan, A. Yaron, A. Berger and M. Sela, Biopolymers, 1965, 3, 625–655 CrossRef CAS PubMed.
- K.-S. Krannig, J. Sun and H. Schlaad, Biomacromolecules, 2014, 15, 978–984 CrossRef CAS PubMed.
- H. Kukula, H. Schlaad, M. Antonietti and S. Förster, J. Am. Chem. Soc., 2002, 124, 1658–1663 CrossRef CAS PubMed.
- R. Sigel, M. Losik and H. Schlaad, Langmuir, 2007, 23, 7196–7199 CrossRef CAS PubMed.
- H. Lu, J. Wang, Y. G. Bai, J. W. Lang, S. Y. Liu, Y. Lin and J. J. Cheng, Nat. Commun., 2011, 2, 206 CrossRef PubMed.
- M. Huo, J. Yuan, L. Tao and Y. Wei, Polym. Chem., 2014, 5, 1519–1528 RSC.
- J. R. Kramer, A. R. Rodriguez, U.-J. Choe, D. T. Kamei and T. J. Deming, Soft Matter, 2013, 9, 3389–3395 RSC.
- D. Pati, N. Kalva, S. Das, G. Kumaraswamy, S. S. Gupta and A. V. Ambade, J. Am. Chem. Soc., 2012, 134, 7796–7802 CrossRef CAS PubMed.
- M. Ambrosi, N. R. Cameron and B. G. Davis, Org. Biomol. Chem., 2005, 3, 1593–1608 CAS.
- X. X. Zeng, T. Murata, H. Kawagishi, T. Usui and K. Kobayashi, Carbohydr. Res., 1998, 312, 209–217 CrossRef CAS.
- A. S. Zarena and S. Gopal, Mini-Rev. Med. Chem., 2013, 13, 1448–1461 CrossRef CAS.
- T. Darbre and J. L. Reymond, Curr. Top. Med. Chem., 2008, 8, 1286–1293 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |


