Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Solution properties and electrospinning of phosphonium gemini surfactants†
Sean T.
Hemp
,
Amanda G.
Hudson
,
Michael H.
Allen
Jr.
,
Sandeep S.
Pole
,
Robert B.
Moore
and
Timothy E.
Long
*
Macromolecules and Interfaces Institute, Department of Chemistry, Virginia Tech, Blacksburg, VA 24061, USA. E-mail: telong@vt.edu; Fax: +1 (540)231-8517; Tel: +1 (540)231-2480
First published on 14th April 2014
Abstract
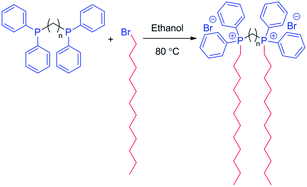
Bis(diphenylphosphino)alkanes quantitatively react with excess 1-bromododecane to prepare novel phosphonium gemini surfactants with spacer lengths ranging from 2 to 4 methylenes (12-2/3/4-12P). Dodecyltriphenylphosphonium bromide (DTPP), a monomeric surfactant analog, was readily water soluble, however, in sharp contrast, phosphonium gemini surfactants were poorly soluble in water due to two hydrophobic tails and relatively hydrophobic cationic head groups containing phenyl substituents. Isothermal titration calorimetry did not reveal a measurable critical micelle concentration for the 12-2-12P phosphonium gemini surfactant in water at 25 °C. Subsequent studies in 50/50 v/v water–methanol at 25 °C showed a CMC of 1.0 mM for 12-2-12P. All phosphonium gemini surfactants effectively complexed nucleic acids, but failed to deliver nucleic acids in vitro to HeLa cells. The solution behavior of phosphonium gemini surfactants was investigated in chloroform, which is an organic solvent where reverse micellar structures are favored. Solution rheology in chloroform explored the solution behavior of the phosphonium gemini surfactants compared to DTPP. The 12-2-12P and 12-3-12P gemini surfactants were successfully electrospun from chloroform to generate uniform fibers while 12-4-12P gemini surfactant and DTPP only electrosprayed to form droplets.
Introduction
Gemini surfactants have received significant attention in the literature recently due to their unique solution properties.1 Gemini surfactants are composed of two conventional monomeric surfactants covalently bound together at their head groups through a spacer.2 The head groups are hydrophilic and composed of ionic3 or nonionic4 groups, and common ionic head groups include ammoniums,5 imidazoliums,6 sulfonates,7 and carboxylates.8 The identity of the spacer directly impacts solution properties9 and a variety of spacers include flexible alkyl or rigid aromatic spacers.10 Gemini surfactants display lower critical micelle concentrations (CMCs),1 enhanced surface active properties,11 and enhanced solubilization12 compared to conventional surfactants. The spacer constrains the head groups, forcing more efficient packing of the head groups into supramolecular assemblies.13 A broad range of supramolecular assemblies include spherical micelles,14 worm-like micelles,15 and vesicles.16Phosphonium gemini surfactants are relatively absent in the earlier literature and to our knowledge, Gin et al.17,18 demonstrated the only example of a phosphonium gemini surfactant in the literature. Using sophisticated techniques, they synthesized bis(alkyl-1,3-diene)-containing phosphonium gemini surfactants with methyl substituents in the head groups. Alkyl tail lengths and spacer lengths directly impacted the self-assembly of the phosphonium gemini surfactants into lyotropic liquid crystalline phases. The terminal diene groups on each tail enabled the crosslinking of the liquid crystalline materials, and the crosslinked gemini surfactants maintained their liquid crystalline phase. Gin and coworkers18 engineered a crosslinked composite containing the phosphonium gemini surfactant to generate a highly efficient water purification membrane.
Candau et al.19 reported three theoretical concentration regimes for surfactants in solution: (1) a dilute regime with slow micellar growth, (2) a semidilute regime wherein the micelles rapidly grow in size, and (3) a concentrated regime where the net charge on the micelle end-caps directly impacts aggregation number. C* and C** correspond to transitions in the concentration regimes from the dilute to semidilute regime or semidilute to concentrated regime, respectively. Long et al.20 examined the solution behavior of a common ammonium gemini surfactant termed 12-2-12A where 2 represents an ethylene spacer with methyl substituents on the cationic head groups. In water, the 12-2-12A surfactant displayed all three concentration regimes and cryo-TEM showed a change in micellar structure between each regime. The scaling factors increased from the dilute regime to the concentrated regime due to enhanced micellar overlap at higher concentrations, leading to higher viscosities. This manuscript strives to directly examine phosphonium gemini surfactants for their solution and electrospinning behavior.
Electrospinning of polymers from solution or melt is a versatile method to generate sub-micron, non-woven fibrous mats suitable for a broad range of applications including filtration and tissue scaffolds.21 During the electrospinning process, the polymer solution is electrified and extruded from a needle whereupon it accelerates towards a grounded or electrified target.22 Charge repulsion within the jet results in stretching and thinning of the fluid until reaching the target and subsequent solidification.23 The polymer must sufficiently stabilize the electrospinning jet to achieve uniform fibers.24 Chain entanglements play a primary role in stabilizing the electrospinning jet, and if the polymer concentration is insufficient to achieve sufficient chain entanglements, electrospraying will occur.25 Long et al.26 first reported the successful electrospinning of a low molar mass surfactant. The phospholipid mixture, soy lecithin, self-assembled into worm-like micelles in organic solution and sufficiently entangled at higher concentrations similar to polymer chains, stabilizing the electrospinning jet and generating uniform fibers. Long et al.20 later extended electrospinning of low molar mass molecules to a common ammonium gemini surfactant (12-2-12A) demonstrating the impact of the supramolecular assembly on electrospinning behavior. Other researchers also have subsequently reported the electrospinning of other small molecules,27–31 however, the electrospinning of phosphonium gemini surfactants remains unprecedented.
We report herein the synthesis and characterization of novel phosphonium gemini surfactants with phenyl-containing head groups, wherein the alkyl spacer between the head groups varied between 2-4 methylenes. Thermogravimetric analysis and differential scanning calorimetry explored the thermal properties of the phosphonium gemini surfactants. The solution behavior of the phosphonium gemini surfactants was examined in both aqueous and organic solutions. Dynamic light scattering and isothermal titration calorimetry examined their aqueous solution behavior. Solution rheology probed the solution behavior of the phosphonium gemini surfactants in organic solvents across a broad concentration regime, and this report describes the successful electrospinning of the phosphonium gemini surfactants to form fibers.
Experimental section
Materials
1,2-Bis(diphenylphosphino)ethane (99%), 1,3-bis(diphenylphosphino)propane (97%), 1,4-bis(diphenylphosphino)butane (98%), dodecyltriphenylphosphonium bromide (98%), and 1-bromododecane (97%) were obtained from Sigma-Aldrich and used as received. All solvents were obtained from Sigma-Aldrich and used as received.Analytical methods
1H and 31P nuclear magnetic resonance (NMR) spectroscopy was performed in CDCl3 using a Varian Unity 400 spectrometer operating at 400 MHz. Mass spectrometry was accomplished using an Agilent 6220 LC-TOF Mass Spectrometer. Thermogravimetric analysis (TGA) was completed using a TA Instruments TGA Q50 operating at a 10 °C min−1 ramp from 25 °C to 600 °C. Differential scanning calorimetry (DSC) was accomplished using a TA Instruments DSC Q1000 with a heat/cool/heat cycle. Solution rheology was completed using a TA Instruments DHR-2 strain-controlled rheometer with a concentric cylinder and solution cup geometry. Zero-shear viscosities were obtained from the Newtonian plateau in shear sweep experiments. Scanning electron microscopy (SEM) was performed using a Nikon Jeol SEM operating at 10 kV under high vacuum. Dynamic light scattering (DLS) was conducted using a Malvern Zetasizer Nano operating at 25 °C.Isothermal titration calorimetry (ITC) measurements were performed with a TA Instruments low volume Nano ITC. The Nano ITC employs a gold reference cell and a sample cell with a fixed volume of 190 μL and a 50 μL gas tight syringe. Duplicate titrations were performed with a sample cell containing ultrapure water, and the injection syringe contained a 0.11 mM solution of sample 12-2-12P. The ITC was maintained at 25 °C with a 350 rpm stir rate and aliquots of 1.43 μL were injected into the sample cell at an interval of 300 s between injections. For measurements with a mixed solvent system, the sample cell was filled with a mixture of 50/50 v/v water–methanol. The injection syringe was filled with an 8.05 mM solution of sample 12-2-12P in the identical 50/50 v/v water–methanol mixture. Injections of the surfactant solution were titrated into the cell in 1.99 μL aliquots with 300 s intervals between each injection. The CMC value was determined as the surfactant concentration at the inflection point of the heat versus concentration profile. The first derivative of Q versus [surfactant] was used to facilitate a precise determination of the inflection point.
Synthesis of 12-2-12P phosphonium gemini surfactant
1,2-Bis(diphenylphosphino)ethane (24.85 g, 62.4 mmol), 1-bromododecane (62.65 g, 251.4 mmol), and ethanol (300 mL) were added to a 500 mL, round-bottomed flask with a magnetic stir bar. The solution was purged with argon for 1 h and subsequently heated at 80 °C for 3 days. The solution was concentrated in vacuo to obtain a viscous liquid. The viscous liquid was added dropwise to 3 L of hexanes to precipitate the gemini surfactant. The gemini surfactant was filtered and washed with 300 mL hexanes three times. The 12-2-12P surfactant was dried in vacuo at 70 °C for 2 days (46.00 g, 82.2% yield). 1H NMR (400 MHz, CDCl3) δ 8.10 (q, 8H, ArH), 7.72 (t, 4H, ArH), 7.64 (t, 8H, ArH), 3.67 (m, 4H, –PCH2CH2P–), 3.58 (m, 4H, –PCH2–), 1.43 (m, 4H, –CH2–), 1.14 (m, 36H, –CH2–), 0.83 (t, 6H, –CH3). 31P NMR (CDCl3) δ 30.68. Mass spectrometry: theoretical, m/z 368.2627; experimental, m/z 368.2659.Synthesis of 12-3-12P phosphonium gemini surfactant
1,3-Bis(diphenylphosphino)propane (21.16 g, 51.3 mmol), 1-bromododecane (53.95 g, 216.5 mmol), and ethanol (300 mL) were added to a 500 mL, round-bottomed flask equipped with a magnetic stir bar. After purging the solution with argon for 1 h, the solution was heated at 80 °C for 3 days. After concentrating the solution in vacuo, the viscous liquid was added to 3 L hexanes to obtain the solid 12-3-12P surfactant. The solid was filtered using a water aspirator and rinsed with 300 mL hexanes three times. The 12-3-12P surfactant was dried in vacuo at 70 °C for 2 days (42.98 g, 92.0% yield). 1H NMR (400 MHz, CDCl3) δ 7.93 (m, 8H, ArH), 7.67 (m, 4H, ArH), 7.61 (m, 8H, ArH), 3.75 (m, 4H, –PCH2), 3.27 (m, 4H, –PCH2–), 2.03 (m, 2H, –CH2–) 1.44 (m, 8H, –CH2–), 1.15 (m, 32H, –CH2–), 0.84 (t, 6H, –CH3). 31P NMR (CDCl3) δ 27.16. Mass spectrometry: theoretical, m/z 375.2706; experimental, m/z 375.2704.Synthesis of 12-4-12P phosphonium gemini surfactant
1,4-Bis(diphenylphosphino)butane (25.04 g, 58.7 mmol), 1-bromododecane (59.64 g, 239.3 mmol), and ethanol (300 mL) were added to a 500 mL, round-bottomed flask containing a magnetic stir bar. The solution was heated to 80 °C for 3 days after purging with argon for 1 h. A majority of the ethanol was removed in vacuo and the viscous liquid was added dropwise to 3 L hexanes. A resulting yellow oil was obtained, which was washed with 3 L hexanes three times to induce solidification. The resulting solid was filtered to obtain the 12-4-12P surfactant. The surfactant was dried in vacuo at 70 °C for 2 days (44.80 g, 82.5% yield). 1H NMR (400 MHz, CDCl3) δ 7.91 (m, 8H, ArH), 7.70 (m, 4H, ArH), 7.65 (m, 8H, ArH), 3.57 (m, 4H, –PCH2), 3.12 (m, 4H, –PCH2–), 1.93 (m, 4H, –CH2–) 1.45 (m, 8H, –CH2–), 1.16 (m, 32H, –CH2–), 0.84 (t, 6H, –CH3). 31P NMR (CDCl3) δ 28.24. Mass spectrometry: theoretical, m/z 382.2784; experimental, m/z 382.2777.Electrospinning
All phosphonium surfactants were electrospun from solutions of chloroform using a syringe pump to control the flow rate at 1 mL h−1. The surfactant solution was electrified at +20 kV with one power supply while the aluminum foil target was electrified at −15 kV using another power supply to generate an overall potential difference of 35 kV. The target was 15 cm from the syringe needle. After electrospinning, the fibers were imaged using SEM and 20 fibers were measured to obtain the average fiber diameter and standard deviation.Polyplex formation and characterization
Phosphonium gemini surfactants displayed poor water solubility, requiring the dissolution of the surfactants in water at 0.1 mg mL−1 concentrations. The heterogenous solutions were sealed and heated to 100 °C for 1 h to generate homogenous solutions and then subsequently cooled to room temperature. DNA gel shift assays were performed as follows. Plasmid DNA (gWiz-Luc, Aldevron, 0.2 μg) was complexed with the required amount of phosphonium gemini surfactant to obtain the desired charge ratio in 28 μL total volume of water for 30 min. Loading buffer (30 wt% glycerol in water, 2 μL) was added and then 20 μL of the polyplex solutions was loaded in a 1 wt% agarose gel stained with 6 μL SYBR Green I (Sigma-Aldrich). The gel was metered at 70 V for 30 min and subsequently imaged using a MultiDoc-it Digital Imaging System (UVP).Cell culture
Human cervical cancer cells (HeLa) were cultured at 37 °C and 5% CO2 in a saturated humid environment. The cells were grown in Dulbecco's modified Eagle's media (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U mL−1 penicillin, and 100 μg mL−1 streptomycin. All reagents were obtained from MediaTech and used as received.Luciferase assay
Polyplex solutions were prepared as follows for transfection. Plasmid DNA (5 μg) was diluted into H2O (250 μL total volume). Phosphonium gemini surfactants (250 μL) at the appropriate concentration to generate the desired charge ratio when added to the DNA solution were also prepared. The surfactant solution was added to the pDNA solution and incubated for 30 min.HeLa cells (500 μL, 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per mL) were plated in 24-well plates 24 h prior to transfection. The complete media was aspirated and the cells were rinsed with 300 μL HBSS. After the addition of 400 μL DMEM to each well, 100 μL of the polyplex solution was added to the well (1 μg DNA per well). Lipofectamine 2000 and Jet-PEI formulations followed recommended manufacturer's protocols. The cells were transfected for 4 h and then the transfection media was aspirated. Complete media (500 μL) was added to each well and the cells were incubated for 44 h. Each well was rinsed with 300 μL PBS and the cells were subsequently lysed with 120 μL 1× Promega lysis buffer. The plates were incubated for 30 min and then subjected to two freeze–thaw cycles. A Promega luciferase kit was utilized following manufacturer's instructions to quantify luciferase expression. Luciferase transfection was reported as relative light units (RLU) and the transfections were performed in quadruplicate. Student's t-test was performed to determine statistical significance at a 95% confidence interval.
000 cells per mL) were plated in 24-well plates 24 h prior to transfection. The complete media was aspirated and the cells were rinsed with 300 μL HBSS. After the addition of 400 μL DMEM to each well, 100 μL of the polyplex solution was added to the well (1 μg DNA per well). Lipofectamine 2000 and Jet-PEI formulations followed recommended manufacturer's protocols. The cells were transfected for 4 h and then the transfection media was aspirated. Complete media (500 μL) was added to each well and the cells were incubated for 44 h. Each well was rinsed with 300 μL PBS and the cells were subsequently lysed with 120 μL 1× Promega lysis buffer. The plates were incubated for 30 min and then subjected to two freeze–thaw cycles. A Promega luciferase kit was utilized following manufacturer's instructions to quantify luciferase expression. Luciferase transfection was reported as relative light units (RLU) and the transfections were performed in quadruplicate. Student's t-test was performed to determine statistical significance at a 95% confidence interval.
Results and discussion
Synthesis
Our group previously reported the synthesis of high molecular weight phosphonium ionenes from the well-defined step-growth polymerization of bis(diphenylphosphino)alkanes and alkyl dibromide monomers.32 A logical extension of this research focused on the synthesis of novel phosphonium gemini surfactants wherein a monofunctional alkyl bromide, 1-bromododecane, was utilized to alkylate the bis(diphenylphosphino)alkanes to generate the desired gemini surfactants. Scheme 1 depicts the synthetic strategy to generate phosphonium gemini surfactants. Gemini surfactants are commonly named x-y-x wherein x corresponds to the alkyl length of the tail and y correlates to the spacer length in between the cationic head groups. 12-y-12P phosphonium gemini surfactants were prepared with y = 2–4 to examine the influence of the alkyl spacer length on solution behavior. All gemini surfactants were synthesized using a 4 molar excess of 1-bromododecane at 80 °C in ethanol for 3 days to ensure complete alkylation and minimal monoalkylation. Dodecyltriphenylphosphonium bromide (DTPP) served as a monofunctional surfactant control for comparison to the phosphonium gemini surfactants.TGA and DSC examined the thermal stability and thermal transitions of the phosphonium gemini surfactants compared to the DTPP control surfactant (Table 1). 12-2-12P gemini surfactant displayed the lowest thermal stability due to the proximity of the phosphonium head groups, which decreased the surfactant thermal stability. Interestingly, 12-3-12P and 12-4-12P gemini surfactants exhibited improved thermal stability compared to DTPP. DSC analysis elucidated both the melting point and glass transition temperature of all surfactants. The melting point was obtained from the first heat, and the glass transition temperature was determined from the second heat. The gemini surfactant Tg decreased as the spacer length increased, and the Tm increased as the spacer length increased.
Gemini surfactants in water
DTPP is readily water soluble and displays a CMC of 1.80 mM in water.33 Phosphonium gemini surfactants displayed poor water solubility, presumably due to the hydrophobic nature of the phenyl-containing head groups and the hydrophobic tails attached to the two head groups. Phosphonium gemini surfactants dissolved in water at 0.1 mg mL−1 concentrations after heating at 100 °C for 1 h. Dynamic light scattering (DLS) experiments probed the solution structure of the phosphonium gemini surfactants in water at 0.1 mg mL−1 concentrations. Fig. 1 highlights the DLS traces for 12-2-12P and 12-3-12P phosphonium gemini surfactants. The size of the aggregates for 12-2-12P and 12-3-12P were 117 ± 2 nm and 105 ± 8 nm, respectively, with monomodal, narrow distributions. 12-4-12P gemini surfactant failed to display well-defined aggregates in aqueous solution based on DLS analysis.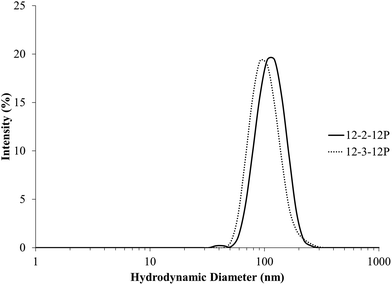 | ||
| Fig. 1 Dynamic light scattering of 12-2-12P and 12-3-12P phosphonium gemini surfactants in 0.1 mg mL−1 aqueous solutions. | ||
Critical micelle concentrations (CMCs) are a fundamental property of all surfactants and multiple techniques including fluorescence spectroscopy,34 conductivity,35 and ITC36 enable the determination of CMCs accurately. Initial experiments to determine the CMC of phosphonium gemini surfactants focused on fluorescence spectroscopy and conductivity methods to determine CMCs. Unfortunately, both methods failed to determine the CMC of phosphonium gemini surfactants. The Krafft temperature, also called the critical micellization temperature, corresponds to the temperature necessary for the surfactant to micellize in solution.37 Below the Krafft temperature, the surfactant fails to display a CMC. The phosphonium gemini surfactants presumably exhibited a very high Krafft temperature due to the hydrophobic tails and hydrophobic phenyl-containing head groups. Furthermore, the process for solution preparation required 100 °C for 1 h to generate 0.1 mg mL−1 gemini surfactant solutions, suggesting a high Krafft temperature. The aqueous phosphonium gemini surfactant solutions were also metastable, and the solutions would become cloudy after extended aging, highlighting a Krafft temperature above room temperature. All experiments were performed shortly after preparing the aqueous solutions to minimize surfactant concentration changes and the effects of aging. Future work will examine the solubility of phosphonium gemini surfactants in aqueous solution to further understand the interplay of the Krafft temperature on micellization behavior.
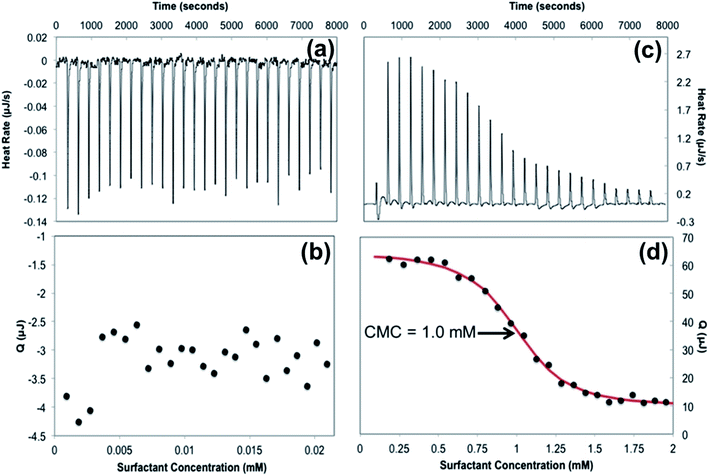
Isothermal titration calorimetry (ITC) is one of the most effective methods to determine the CMC of a surfactant along with other thermodynamic information regarding the micellization behavior.36Fig. 2 depicts ITC experiments performed for 12-2-12P in water and a 50/50 v/v water–methanol solution. The titration of a concentrated 12-2-12P aqueous solution into water resulted in titration curves similar to the titration of water into water suggesting the absence of appreciable demicellization in pure water at 25 °C. This behavior is again consistent with the presumed high Krafft temperature for these gemini surfactants. In contrast, titrations performed in 50/50 v/v water–methanol resulted in titration curves that are representative of conventional surfactant CMC behavior, as shown in Fig. 2d. Based on this unique ITC approach, the CMC for 12-2-12P in 50/50 v/v water–methanol was found to be 1.0 mM at 25 °C. The addition of methanol resulted in improved solubility of the phosphonium gemini surfactants and enabled the determination of the CMC using ITC. Further work will develop a method to extrapolate the CMC of phosphonium gemini surfactants to that in pure water through a series of ITC experiments using variable concentrations of water–methanol.
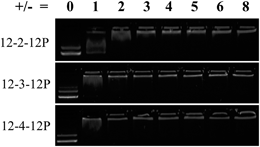
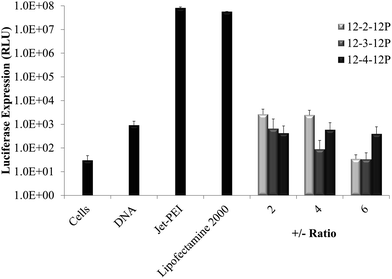
DNA gel shift assays and luciferase transfection assays examined the suitability of phosphonium gemini surfactants as nonviral nucleic acid delivery agents. The complexation of a cationic surfactant and negatively-charged nucleic acid in solution generates nanoparticles or aggregates called lipoplexes. Fig. 3 shows DNA gel shift assays for all phosphonium gemini surfactants, which confirmed the efficacy of phosphonium gemini surfactants to bind nucleic acids. The charge ratio (±ratio) was defined as the moles of cationic head groups in the surfactant to the moles of negatively-charged phosphate groups in the plasmid DNA backbone. All phosphonium gemini surfactants bound nucleic acids with 12-2-12P, 12-3-12P, and 12-4-12P binding plasmid DNA completely at ±ratios of 3, 2, and 2, respectively. Luciferase transfection experiments in serum-free media elucidated low transfection efficiency of phosphonium gemini surfactants compared to common positive controls, Jet-PEI and Lipofectamine 2000 (Fig. 4).
Gemini surfactants in chloroform
The goal of generating electrospun fibers composed of the phosphonium gemini surfactants required an examination of the solution behavior of phosphonium gemini surfactants in organic solutions to provide information necessary for electrospinning. Phosphonium gemini surfactants displayed excellent solubility in organic solvents except for hexanes. Chloroform was chosen as the solvent to examine the solution properties of phosphonium gemini surfactants due to its ability to dissolve the surfactants and its necessary volatility for electrospinning. Surfactants typically generate reverse micelles and other reverse supramolecular structures in organic solutions.38,39 Reverse micelles differ from normal micelles in that the head group sequesters itself into the micelle core and the hydrophobic tails reside in the shell of the micelle.Solution rheology of DTPP and all phosphonium gemini surfactants studied the solution behavior of the surfactants in chloroform. Fig. 5 reports the specific viscosity vs. concentration for all surfactants. In each plot, three different solution regimes were identified based upon a change in the slope of the data. C* and C** were defined as the crossover points where each power law regression overlapped with the power law regression of the adjacent concentration regime. All surfactants displayed similar C* transitions, occurring at ∼19 wt% in chloroform. C** depended significantly on the surfactant with DTPP displaying significantly a higher C** at 39 wt% compared to 30–32 wt% for the phosphonium gemini surfactants. The constraint of the head groups in the gemini surfactants presumably resulted in different supramolecular structures, which resulted in significant overlap of the structures in solution at lower concentrations. Soy lecithin, a phospholipid mixture, displayed a similar C** of 35 wt% in 70/30 v/v chloroform–DMF compared to the phosphonium gemini surfactants.26
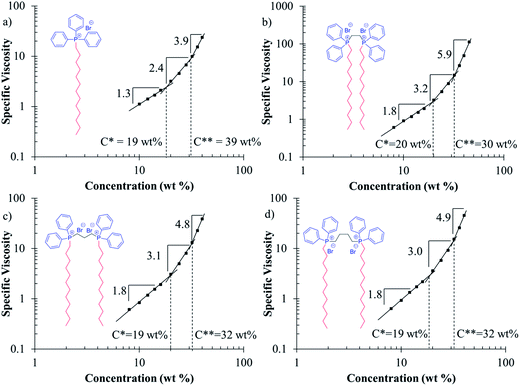 | ||
| Fig. 5 Solution rheology of phosphonium control surfactant and phosphonium gemini surfactants in chloroform. | ||
Polymer solution rheology utilizes the scaling parameters in each concentration regime to identify the solution behavior of the polymer. For instance, neutral, non-associating polymers display significantly different scaling factors compared to polyelectrolytes and the scaling factors provide information regarding the polymer solution conformation and solution behavior.40 All phosphonium gemini surfactants exhibited higher scaling factors compared to the DTPP control, especially in the concentrated regime above C**. 12-2-12P gemini surfactant resulted in the highest scaling factors compared to all surfactants, likely due to the short ethylene spacer constraining the conformation of the head group. As a comparison, soy lecithin in 70/30 v/v chloroform–DMF displayed scaling factors of 2.4 and 8.4 in the semidilute and concentrated regimes, respectively.26 The phosphonium gemini surfactants exhibited similar semidilute scaling factors as soy lecithin, but their concentrated scaling factors were significantly lower than the concentrated scaling factor for soy lecithin.
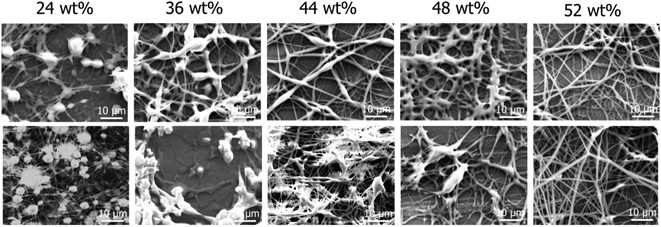
Attempts at electrospinning DTPP and the phosphonium gemini surfactants examined the impact of the surfactant structure on fiber formation. All electrospinning attempts occurred at concentrations of 52 wt% or lower. DTPP and 12-4-12P gemini surfactant failed to generate any electrospun fibers, primarily electrospraying droplets. Conversely, 12-2-12P and 12-3-12P gemini surfactants sufficiently stabilized the electrospinning jet to generate electrospun fibers as shown in Fig. 6. At concentrations less than 52 wt%, ill-defined fibers were formed with the incorporation of droplets or beaded fibers. Concentrations of 52 wt% were necessary to generate uniform, well-defined fibers. The 12-2-12P and 12-3-12P gemini surfactants generated fibers diameters of 1.0 ± 0.3 μm and 1.1 ± 0.2 μm, respectively, at 52 wt% in chloroform. As a comparison, aqueous solutions (50/50 v/v water–methanol) of an ammonium gemini surfactant (12-2-12A) at concentrations of 42 and 44 wt% generated uniform fibers of 4 and 5 μm diameters, respectively.20 Ultimately, spacers of 2 or 3 methylenes were necessary to generate a stable electrospinning jet to obtain uniform fibers. The longer alkyl spacer of 4 methylenes and the DTPP surfactant failed to generate electrospun fibers, presumably due to the absence of sufficient supramolecular entanglements.
Conclusions
The alkylation of ditertiary phosphines using 1-bromododecane generated phosphonium gemini surfactants in high yields. The cationic head groups displayed significant hydrophobicity due to the phenyl substituents leading to poor solubility in water. 12-2-12P and 12-3-12P gemini surfactants self-assembled into large supramolecular structures as shown with DLS. Due to limited solubility, isothermal titration calorimetry was ineffective in the determination of the CMC for 12-2-12P in pure water at 25 °C. As an alternative new ITC approach, the CMC of the 12-2-12P gemini surfactant was determined to be 1.0 mM at 25 °C in a mixed solvent of 50/50 v/v water–methanol. Preliminary biological assays focused on nucleic acid complexation, and demonstrated efficient nucleic acid binding for phosphonium gemini surfactants. However, the phosphonium gemini surfactants failed to efficiently deliver nucleic acids in vitro to HeLa cells. Phosphonium gemini surfactants were also studied in chloroform that promoted reverse micellar structures. Solution rheology elucidated the overall solution behavior of phosphonium gemini surfactants and DTPP in chloroform. Ultimately, electrospinning of 12-2-12P and 12-3-12P gemini surfactants generated uniform fibers with fiber diameters of ∼1 μm while 12-4-12P and DTPP failed to electrospin.Acknowledgements
This material is based upon work supported in part by the U.S. Army Research Office under grant number W911NF-07-1-0452 Ionic Liquids in Electro-Active Devices (ILEAD) MURI. This material is based upon work supported in part by the U.S. Army Research Laboratory and the U.S. Army Research Office under the Army Materials Center of Excellence Program, contract W911NF-06-2-0014. This material is partially based upon work supported by the National Science Foundation under Grant no. DMR-0923107. We acknowledge funding from NSF (CHE-0722638) for the acquisition of our Agilent 6220 LC-TOF-MS. This material is based upon work supported by the Army Research Office (ARO) under Award no. W911NF-10-1-0307 (DURIP). The authors also acknowledge the Institute for Critical Technology and Applied Science (ICTAS) at Virginia Tech for facility support.References
- F. M. Menger and J. S. Keiper, Angew. Chem., Int. Ed., 2000, 39, 1906–1920 CrossRef.
- F. M. Menger and C. Littau, J. Am. Chem. Soc., 1991, 113, 1451–1452 CrossRef CAS.
- F. Menger and C. Littau, J. Am. Chem. Soc., 1993, 115, 10083–10090 CrossRef CAS.
- M. J. Castro, J. Kovensky and A. Fernández Cirelli, Langmuir, 2002, 18, 2477–2482 CrossRef CAS.
- X. Wang, J. Wang, Y. Wang, H. Yan, P. Li and R. K. Thomas, Langmuir, 2003, 20, 53–56 CrossRef.
- M. Ao, G. Xu, Y. Zhu and Y. Bai, J. Colloid Interface Sci., 2008, 326, 490–495 CrossRef CAS PubMed.
- Y. Wang, Y. Han, X. Huang, M. Cao and Y. Wang, J. Colloid Interface Sci., 2008, 319, 534–541 CrossRef CAS PubMed.
- X. Huang, M. Cao, J. Wang and Y. Wang, J. Phys. Chem. B, 2006, 110, 19479–19486 CrossRef CAS PubMed.
- M. J. Rosen and L. D. Song, J. Colloid Interface Sci., 1996, 179, 261–268 CrossRef CAS.
- L. D. Song and M. J. Rosen, Langmuir, 1996, 12, 1149–1153 CrossRef CAS.
- M. J. Rosen, J. H. Mathias and L. Davenport, Langmuir, 1999, 15, 7340–7346 CrossRef CAS.
- M. Dreja and B. Tieke, Langmuir, 1998, 14, 800–807 CrossRef CAS.
- T. A. Camesano and R. Nagarajan, Colloids Surf., A, 2000, 167, 165–177 CrossRef CAS.
- M. Bergsma, M. L. Fielden and J. B. F. N. Engberts, J. Colloid Interface Sci., 2001, 243, 491–495 CrossRef CAS.
- R. Oda, P. Panizza, M. Schmutz and F. Lequeux, Langmuir, 1997, 13, 6407–6412 CrossRef CAS.
- M. Johnsson, A. Wagenaar and J. B. F. N. Engberts, J. Am. Chem. Soc., 2002, 125, 757–760 CrossRef PubMed.
- B. A. Pindzola, J. Jin and D. L. Gin, J. Am. Chem. Soc., 2003, 125, 2940–2949 CrossRef CAS PubMed.
- M. Zhou, P. R. Nemade, X. Lu, X. Zeng, E. S. Hatakeyama, R. D. Noble and D. L. Gin, J. Am. Chem. Soc., 2007, 129, 9574–9575 CrossRef CAS PubMed.
- F. Kern, F. Lequeux, R. Zana and S. J. Candau, Langmuir, 1994, 10, 1714–1723 CrossRef CAS.
- M. P. Cashion, X. Li, Y. Geng, M. T. Hunley and T. E. Long, Langmuir, 2009, 26, 678–683 CrossRef PubMed.
- Z.-M. Huang, Y.-Z. Zhang, M. Kotaki and S. Ramakrishna, Compos. Sci. Technol., 2003, 63, 2223–2253 CrossRef CAS.
- D. H. Reneker, A. L. Yarin, H. Fong and S. Koombhongse, J. Appl. Phys., 2000, 87, 4531–4547 CrossRef CAS PubMed.
- M. G. McKee, M. T. Hunley, J. M. Layman and T. E. Long, Macromolecules, 2005, 39, 575–583 CrossRef.
- S. L. Shenoy, W. D. Bates, H. L. Frisch and G. E. Wnek, Polymer, 2005, 46, 3372–3384 CrossRef CAS PubMed.
- M. G. McKee, G. L. Wilkes, R. H. Colby and T. E. Long, Macromolecules, 2004, 37, 1760–1767 CrossRef CAS.
- M. G. McKee, J. M. Layman, M. P. Cashion and T. E. Long, Science, 2006, 311, 353–355 CrossRef CAS PubMed.
- J. C. Singer, R. Giesa and H.-W. Schmidt, Soft Matter, 2012, 8, 9972–9976 RSC.
- M. Chen, S. R. Nielsen, T. Uyar, S. Zhang, A. Zafar, M. Dong and F. Besenbacher, J. Mater. Chem. C, 2013, 1, 850–855 RSC.
- A. Celebioglu and T. Uyar, J. Colloid Interface Sci., 2013, 404, 1–7 CrossRef CAS PubMed.
- G. Singh, A. M. Bittner, S. Loscher, N. Malinowski and K. Kern, Adv. Mater., 2008, 20, 2332–2336 CrossRef CAS.
- X. Yan, M. Zhou, J. Chen, X. Chi, S. Dong, M. Zhang, X. Ding, Y. Yu, S. Shao and F. Huang, Chem. Commun., 2011, 47, 7086–7088 RSC.
- S. T. Hemp, M. Zhang, M. Tamami and T. E. Long, Polym. Chem., 2013, 4, 3582–3590 RSC.
- M. Prasad, S. P. Moulik, A. MacDonald and R. Palepu, J. Phys. Chem. B, 2003, 108, 355–362 CrossRef.
- K. Kalyanasundaram and J. Thomas, J. Am. Chem. Soc., 1977, 99, 2039–2044 CrossRef CAS.
- M. Pérez-Rodríguez, G. Prieto, C. Rega, L. M. Varela, F. Sarmiento and V. Mosquera, Langmuir, 1998, 14, 4422–4426 CrossRef.
- S. P. Stodghill, A. E. Smith and J. H. O'Haver, Langmuir, 2004, 20, 11387–11392 CrossRef CAS PubMed.
- Z. Chu and Y. Feng, Langmuir, 2011, 28, 1175–1181 CrossRef PubMed.
- P. K. Das and A. Chaudhuri, Langmuir, 1999, 16, 76–80 CrossRef.
- P. Schurtenberger, R. Scartazzini, L. J. Magid, M. E. Leser and P. L. Luisi, J. Phys. Chem., 1990, 94, 3695–3701 CrossRef CAS.
- R. Colby, Rheol. Acta, 2010, 49, 425–442 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4sm00271g |
| This journal is © The Royal Society of Chemistry 2014 |