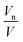Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The effects of pressure and temperature on the energetics and pivotal surface in a monoacylglycerol/water gyroid inverse bicontinuous cubic phase†
T.-Y. Dora
Tang
*ab,
Annela M.
Seddon
ac,
Christoph
Jeworrek
d,
Roland
Winter
d,
Oscar
Ces
a,
John M.
Seddon
a and
Richard H.
Templer
a
aDepartment of Chemistry, Imperial College London, Exhibition Road, London, SW7 2AY, UK
bSchool of Chemistry, University of Bristol, Cantock's Close, Bristol BS8 1TS, UK. E-mail: chtydt@bristol.ac.uk
cHH Wills Physics Laboratory, University of Bristol, Tyndall Avenue, BS8 1FD, UK
dPhysical Chemistry I—Biophysical Chemistry, Faculty of Chemistry, Technische Universität Dortmund, Otto-Hahn-Str. 6, 44227 Dortmund, Germany
First published on 20th February 2014
Abstract
We have studied the effect of pressure and temperature on the location of the pivotal surface in a lipid inverse bicontinuous gyroid cubic phase (QGII), described by the area at the pivotal surface (An), the volume between the pivotal surface and the bilayer midplane (Vn), and the molecular volume of the lipid (V). Small angle X-ray scattering (SAXS) was used to measure the swelling behaviour of the lipid, monolinolein, as a function of pressure and temperature, and the data were fitted to two different geometric models: the parallel interface model (PIM), and the constant mean curvature model (CMCM). The results show that an increase in temperature leads to a shift in the location of the pivotal surface towards the bilayer midplane, whilst an increase in pressure causes the pivotal surface to move towards the interfacial region. In addition, we describe the relevance of An, Vn and V for modeling the energetics of curved mesophases with specific reference to the mean curvature at the pivotal surface and discuss the significance of this parameter for modelling the energetics of curved mesophases.
Introduction
Liquid crystalline phases with curved interfaces, such as the inverse bicontinuous cubic phases (QII), are mathematically and geometrically elegant. The most well known and characterized of the inverse bicontinuous phases are based upon the diamond, gyroid and primitive triply periodic minimal surfaces.1,2 In the liquid crystalline phase QDII (diamond), QGII (gyroid) and QPII (primitive) the midplane of an amphiphilic bilayer lies upon a diamond, gyroid or primitive minimal surface of zero mean curvature and subdivides three dimensional space to form intertwined but non-penetrating water channels. Each inverse bicontinuous cubic phase differs in the connectivity of these water channels, for example, in the QGII phase the channels are connected 3-fold at 120° (Fig. 1), whilst the junctions in the QDII phase connect 4-fold at 109° and 6-fold at 90° for the QPII phase.The formation of these curved mesophases from amphiphilic molecules such as surfactants, polymers and lipids are driven by (i) the hydrophobic effect,3 and (ii) a balance between the curvature elastic stress and packing energy.4 When curvature elastic stress drives the deformation of a flat bilayer to induce curvature there is a large energetic cost associated with the formation of hydrophobic voids which can be compensated by the stretching of hydrocarbon chains within the bilayer. The balance of curvature stress and packing energy leads to an energy minimum which favours the stable formation of the inverse bicontinuous phases. Experimentally it has been shown that the hydration, temperature, pressure, pH and the molecular structure of the lipid5–12 can tune the formation of the QII phases. As a consequence, these structures have been exploited in technologies ranging from drug delivery11,13,14 and renewable energies7,15–17 to protein crystallography12,18–20 and gene silencing21 where the large surface area to volume ratio, high water content and controllable and tuneable structural properties have been exploited. However, a complete theoretical and energetic description of inverse bicontinuous phases is still lacking and this is essential for rational design of curved mesophases for technological applications.
One of the difficulties in modeling the QII phases is attributed to its curved bilayer as the curvature and therefore the energetics of the phase are dependent on the transverse location within the bilayer from which it is measured.22 However, within the curved bilayer there exists a surface, the pivotal surface, whose area remains constant as the bilayer bends with the swelling of the unit cell, thus the energy can be defined at this surface and its location can be obtained by modeling isothermal and isobaric swelling data.23,24
The pivotal surface of a monolayer is defined by three parameters; An, the area at the pivotal surface; Vn, the volume between the bilayer midplane and the pivotal surface; and V, the molecular volume (Fig. 1c).  is directly related to the position of the pivotal surface in the lipid bilayer. V can be experimentally obtained by measuring the density of the lipid whilst An and Vn are found by fixing the geometry of the interface. For the case of the inverse bicontinuous cubic phases, this interface can be defined by two opposing geometric models (Fig. 1b). The first (historically) is the parallel interface model (PIM)25 where the pivotal surface is constrained to lie equidistant from the bilayer midplane. In the second geometry, the Constant Mean Curvature Model (CMCM), the interface adopts a constant mean curvature.26–28 Detailed theory behind each of these models is given in Appendix A. Constraining the interface inevitably leads to molecular frustration within the bilayer; for example, curvature stress will dominate when a monolayer is constrained to a parallel interface. Conversely, packing frustration will dominate when the bilayers are constrained to a constant mean curvature. Therefore, a balance of energetic contributions from packing frustration and curvature stress is believed to lead to the stable formation of the inverse bicontinuous cubic phases;29 however the exact contributions from frustration and stress are not fully understood and this is a non-trivial matter.
is directly related to the position of the pivotal surface in the lipid bilayer. V can be experimentally obtained by measuring the density of the lipid whilst An and Vn are found by fixing the geometry of the interface. For the case of the inverse bicontinuous cubic phases, this interface can be defined by two opposing geometric models (Fig. 1b). The first (historically) is the parallel interface model (PIM)25 where the pivotal surface is constrained to lie equidistant from the bilayer midplane. In the second geometry, the Constant Mean Curvature Model (CMCM), the interface adopts a constant mean curvature.26–28 Detailed theory behind each of these models is given in Appendix A. Constraining the interface inevitably leads to molecular frustration within the bilayer; for example, curvature stress will dominate when a monolayer is constrained to a parallel interface. Conversely, packing frustration will dominate when the bilayers are constrained to a constant mean curvature. Therefore, a balance of energetic contributions from packing frustration and curvature stress is believed to lead to the stable formation of the inverse bicontinuous cubic phases;29 however the exact contributions from frustration and stress are not fully understood and this is a non-trivial matter.
Previous studies have shown that the monoacylglycerols form a range of inverse bicontinuous cubic phases as a function of pressure and temperature6,7,11,30,31 and their well characterized experimental phase behavior make them ideal models for measuring the pivotal surface parameters. However, the PIM and the CMCM are limited at low water contents where the molecular deformations within the monolayer increase and the bilayer can no longer maintain a fixed geometry. As the QGII phase formed by monolinolein is hydrated between 15 wt% water and 30 wt% water, determining the pivotal surface parameters in this system should not be affected by the limits of the geometric models.
Here we use the two geometric models, the PIM and the CMCM, to model the location of the pivotal surface in the QGII phase, as a function of pressure and temperature, in the binary monolinolein (ML)–water system. The swelling behavior of ML as a function of temperature and pressure was characterized using SAXS, and An and Vn were obtained from fitting PIM and CMCM to the swelling data. Having obtained An and Vn the mean curvature at the pivotal surface was calculated using the constant mean curvature model, and the validity of the geometrical models for obtaining structural parameters in curved lipid bilayers is discussed.
Materials and methods
Sample preparation
Lyophilised monolinolein (ML) (Larodan, Switzerland) was mixed with a known amount of HPLC grade water (Sigma Aldrich, UK) to the desired wt% water. The weight of the lipid and water was measured to a precision of 6 decimal places. Sample homogeneity was achieved by subjecting the lipid–water mixture to at least 50 freeze–thaw cycles (−70 °C to 20 °C).For temperature dependent experiments, samples were prepared directly into 1.5 mm special glass capillaries. Once the lipid and water were loaded into the glass capillary the capillary was heat sealed and further sealed with silicone sealant and subjected to freeze–thaw cycles. These were stored at −20 °C until the experiments were carried out. For pressure dependent experiments the samples were prepared in glass vials as described above and stored at −20 °C. Immediately before the experiment the lipid–water mixture was transferred to a Teflon ring enclosed by two mylar foils which isolated the sample from the external environment. The deviation from the weighed water content and the actual water content was typically 1%; this error is attributed to water loss during sample transfer to the Teflon spacers and is typical of this experimental procedure.
X-ray scattering measurements
Temperature dependent experiments were undertaken using an in-house Bede Microscource™ X-ray generator (Durham, UK). Temperature was controlled by an external water bath; the temperature of the sample holder is controlled via a Pt100 (TC limited, Uxbridge, UK) temperature probe connected to a microcontroller which has the capability of maintaining the temperature to ±0.1 °C. X-ray diffraction images were acquired using an image intensified charge-coupled device X-ray camera (Gemstar HS, Photonic Science Ltd, UK). Typically, five 30 second exposures were taken at each temperature and summed using the custom built software package AXcess.32 Images were acquired at 2 °C intervals from 0 °C to 70 °C with a 10 minute equilibration at each temperature. Lattice parameter values (in Å) were calibrated using silver behenate (a = 58.38 Å) and AXcess was used to characterise the phase and to determine the lattice parameter to an accuracy of ±0.05 Å.Pressure dependent experiments were performed at beamline ID02 at the European Synchroton Radiation Facility (ESRF), Grenoble, France and I22 at Diamond Light Source (DLS), Oxfordshire, UK using a high pressure diamond cell16 constructed from Ni–Cr–Co alloy (NIMONIC 90) capable of withstanding hydrostatic pressures of up to 3000 bar generated by a manual pumping system. The pump and high pressure tubing and valves were purchased from Nova Swiss, Effretikon, Switzerland. Temperature was controlled via circulating water from an external water bath (accurate ±0.2 °C) through the jacket of the cell and measured by a thermocouple embedded into the body of the cell. The high pressure cell is equipped with diamond windows glued into metal supports and X-ray transmission through the diamond windows was approximately 65% using X-rays of energy 17 keV (λ = 0.75 Å). At beamline ID02, ESRF, a maximum flux of 4 × 1013 photons per second was achieved. To achieve sample equilibration the samples were subjected to 10 pressure cycles from 1 bar to 2000 bar and samples were left for approximately 30 minutes after a change in temperature, and at least 2 min at each pressure before capturing a X-ray diffraction image with typical exposure times of 0.1 s. X-ray diffraction images were taken using a Frelon (Fast readout, low noise) Kodak CCD detector with a maximum frame rate of 15 frames per s. Diffraction patterns were analyzed off line using AXcess software.
Lipid density measurements
The density of limited hydration monolinolein, over a range of temperatures and pressures, was measured using an Anton Paar DMA 60/512P (Graz Austria) vibrating tube densimeter with a steel U-tube of internal volume 2.5 ml connected to a high pressure network capable of achieving pressures up to 700 bar. Temperature was controlled via a recirculating water bath, accurate to 0.1 °C.Three 1 g monolinolein–water samples were prepared with 23 wt% water using degassed Milli-Q water. Samples were mechanically mixed via centrifugation and then subjected to more than 40 freeze–thaw cycles to ensure sample homogeneity. The individual portions were combined and freeze-thawed a further 20 times, then stored under nitrogen at −20 °C until required. Samples were loaded into the U-tube using a custom built apparatus to minimize the number of air bubbles in the sample. Lipid density was measured at 11 °C, 17 °C, 24 °C, 37 °C from 0–700 bar at 100 bar intervals.
To obtain an accurate water content of the lipid–water mixture the sample was removed from the U-tube after the experiment, weighed, and then re-weighed after 48 hours of lyophilisation. There was a 1% difference in water content compared to the expected water content, which was attributed to lipid loss during mechanical mixing or sample transfer.
The molecular volume was obtained from the lipid density as a function of pressure and temperature. Molecular volumes at intermediate temperatures and pressures were obtained via extrapolation of the experimental data (Table 1 ESI†).
Modelling the energetics of the inverse bicontinuous phases using geometric models
 | (1) |
 | (2) |
 .33 Relating the volume fraction ϕw, V, An and Vn to the lattice parameter, a, the constant mean curvature model is given for QGII by eqn (3), with σ0 = 3.0915; σ1 = −1.3317; σ2 = −0.19974; σ3 = −0.80113.
.33 Relating the volume fraction ϕw, V, An and Vn to the lattice parameter, a, the constant mean curvature model is given for QGII by eqn (3), with σ0 = 3.0915; σ1 = −1.3317; σ2 = −0.19974; σ3 = −0.80113. | (3) |
 | (4) |
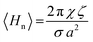 | (5) |
Previous studies have shown that the constant mean curvature model is the most appropriate model for describing the curved interfaces of the inverse bicontinuous phases,34 therefore a can be described by eqn (3) and the surface averaged mean curvature, defined by the CMCM, is:
 | (6) |
By determining the effects of pressure and temperature on the lattice parameter and consequently the pivotal surface, it is possible to make semi-quantitative statements about the effect of pressure, temperature or water content on the stability of the QGII phase.
Data fitting
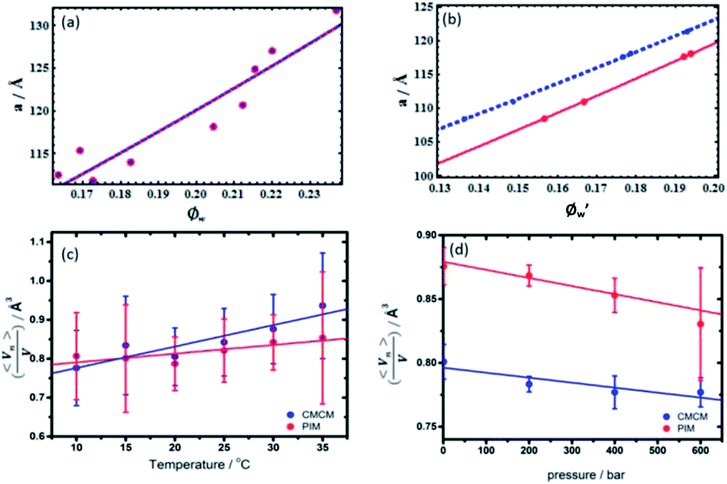
 (Fig. 2). To reduce the experimental error incurred by preparation of large volumes of limited hydrated monolinolein, the models were fitted to a recalculated water content, Ø′w, to obtain 〈An〉 and
(Fig. 2). To reduce the experimental error incurred by preparation of large volumes of limited hydrated monolinolein, the models were fitted to a recalculated water content, Ø′w, to obtain 〈An〉 and 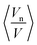 . The recalculated water content for each sample was obtained from the corresponding lattice parameter at 1 bar and 30 °C using eqn (1) and (2) where An, Vn and V were obtained by fitting the models to isobaric data obtained at 30 °C as the water content at different pressures and temperatures is constant for a homogeneous sample. Whilst eliminating the error between the actual water content and the expected water content this statistical treatment meant that the swelling data at constant temperature was constrained to a fixed geometry, therefore it is only possible to determine the qualitative effect of pressure on the pivotal surface.
. The recalculated water content for each sample was obtained from the corresponding lattice parameter at 1 bar and 30 °C using eqn (1) and (2) where An, Vn and V were obtained by fitting the models to isobaric data obtained at 30 °C as the water content at different pressures and temperatures is constant for a homogeneous sample. Whilst eliminating the error between the actual water content and the expected water content this statistical treatment meant that the swelling data at constant temperature was constrained to a fixed geometry, therefore it is only possible to determine the qualitative effect of pressure on the pivotal surface.
Results and discussion
Swelling data obtained from SAXS experiments at constant pressure, from 10 °C to 35 °C and recalculated water contents at constant temperature, 30 °C from 1 bar to 600 bar were fit to the PIM and CMCM using the molecular volume, V, obtained from density measurements (ESI Table S1†) to obtain the pivotal surface parameters An and Vn as a function of temperature and pressure.The results showed that there was a good fit to isobaric data with a R2 ≥ 0.999999 and to isothermal data where R2 ≥ 0.999 confirming that the models are a valid description of the pivotal surface in the QGII phase under these conditions. The precise interfacial geometry of the bicontinuous cubic phases is not known, but it has been modelled in two ways26 – an interface of uniform thickness (the parallel interface model) and one with constant mean curvature (the constant mean curvature model). The parallel interface model makes geometric calculations straightforward, but has the unfortunate characteristic that bicontinuous cubic phases, such as QGII, QDII and QPII, which are related by a Bonnet transformation, exhibit energetic degeneracy for any energetic term that depends on interfacial geometry.35 This energetic degeneracy does not exist when the interface has constant mean curvature and it has been shown that the qualitative characteristics of the phase behaviour of the three bicontinuous cubic phases are replicated. For example, replication of the phase sequence from QGII → QDII → QPII with increasing water content and negative phase boundaries are observed.34 Despite this, this geometric model has its own limitations – the interfaces become self-intersecting at reduced hydrations, where the mesophases are still found to exist in nature.36,37 Within these limitations both models are able to give us insight into the behaviour of these lyotropic structures as thermodynamic conditions are varied. Our studies showed that comparisons between the geometric fits showed no difference, within error, in the pivotal surface parameters between 10 °C and 35 °C (Fig. 3 and S1†) and this is attributed to small contributions of chain stretching to the energy of the QGII phase in monolinolein when Øw lies between 0.12 and 0.25. These results suggest that under these conditions, both geometric models are suitable for determining the pivotal surface parameters for the QGII phase, indicating that there are contributions from curvature elastic stress and packing frustration to the energetics of the QGII phase in limited hydration monolinolein. Determining the precise contributions of the curvature elastic energy and packing frustration is a non-trivial matter which could be achieved if the error in experiment was less than the difference in contributions from curvature stress and packing frustration. At atmospheric pressure and 25 °C,  which positions the pivotal surface between carbon 1 and 2 of the acyl chain. This is comparable to
which positions the pivotal surface between carbon 1 and 2 of the acyl chain. This is comparable to  obtained using the PIM in monoolein23 and didodecyl-b-D-glucopyranosyl38 of 0.76 and 0.83 respectively and corresponds to the position in the acyl chain where the lateral compressibility is at its lowest and therefore where the interface will pivot. Our results show that the position of the pivotal surface is linearly dependent on temperature and pressure. Increasing the temperature shifts the pivotal surface away from the bilayer midplane by 0.2% K−1 and increasing the pressure moves the pivotal surface towards the bilayer midplane by approximately 6% kbar−1. Previous studies showed that
obtained using the PIM in monoolein23 and didodecyl-b-D-glucopyranosyl38 of 0.76 and 0.83 respectively and corresponds to the position in the acyl chain where the lateral compressibility is at its lowest and therefore where the interface will pivot. Our results show that the position of the pivotal surface is linearly dependent on temperature and pressure. Increasing the temperature shifts the pivotal surface away from the bilayer midplane by 0.2% K−1 and increasing the pressure moves the pivotal surface towards the bilayer midplane by approximately 6% kbar−1. Previous studies showed that 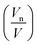 was constant with temperature23 and deconvolution of the effects of pressure and temperature on the pivotal surface were attributed to greater precision in the experimental data afforded by technological advances in bench top X-rays and 3rd generation synchrotron sources. The sensitivity of the location of the pivotal surface to changes in temperature and pressure can be understood by considering the lateral stresses within the monolayer as the thermodynamic parameters are altered. For example, increasing the temperature increases the positive lateral stresses in the acyl chain region due to an increase in the number of trans-gauche rotamers in the hydrocarbon chain region whilst increasing the pressure leads to a decrease in positive lateral stresses as the hydrocarbon chain is compressed. Indeed, calculation of the compressibility of the acyl chain region between the pivotal surface and the bilayer midplane and the pivotal surface (Bad) from experimental data (see ESI†) gave 7.15 × 10−10 Pa−1 (CMCM) which is comparable to the adiabatic compressibility of linoleic acid, 6.90 × 10−10 Pa−1 at 65 °C, obtained by ultrasound experiments.15 This indicates that the compressibility in the acyl chain, affected by changes in temperature and pressure, will lead to changes in the lateral stresses within the bilayer. Consequently the pivotal surface will move transverse to the bilayer midplane to maintain a balance of forces on either side of the pivotal surface.
was constant with temperature23 and deconvolution of the effects of pressure and temperature on the pivotal surface were attributed to greater precision in the experimental data afforded by technological advances in bench top X-rays and 3rd generation synchrotron sources. The sensitivity of the location of the pivotal surface to changes in temperature and pressure can be understood by considering the lateral stresses within the monolayer as the thermodynamic parameters are altered. For example, increasing the temperature increases the positive lateral stresses in the acyl chain region due to an increase in the number of trans-gauche rotamers in the hydrocarbon chain region whilst increasing the pressure leads to a decrease in positive lateral stresses as the hydrocarbon chain is compressed. Indeed, calculation of the compressibility of the acyl chain region between the pivotal surface and the bilayer midplane and the pivotal surface (Bad) from experimental data (see ESI†) gave 7.15 × 10−10 Pa−1 (CMCM) which is comparable to the adiabatic compressibility of linoleic acid, 6.90 × 10−10 Pa−1 at 65 °C, obtained by ultrasound experiments.15 This indicates that the compressibility in the acyl chain, affected by changes in temperature and pressure, will lead to changes in the lateral stresses within the bilayer. Consequently the pivotal surface will move transverse to the bilayer midplane to maintain a balance of forces on either side of the pivotal surface.
In order to determine the effects of pressure and temperature on the curvature elastic energy, the pivotal surface parameters obtained from experimental data were used to calculate Hn as a function of water content, pressure and temperature, using the constant mean curvature model. The results showed a non-linear dependence on Hn with a decreasing negative curvature with increasing water content at all measured temperatures and pressures. Assuming that 〈Kn〉 is minimized, a decrease in Hn with increasing negative curvature describes a reduction in the asymmetric molecular deformation of the lipid shape. This is consistent with previous results which underpins characteristic phase transitions from QGII to QDII to QPII with increasing water content for this class of lipids,34 and shows that the quadratic dependence of curvature elastic energy on Hn removes the energetic degeneracy between the three inverse bicontinuous cubic phases. Increasing the temperature caused Hn to increase in magnitude, whilst increasing the pressure led to a decrease in magnitude in Hn. These qualitative trends are consistent with observations from experimental phase diagrams for monolinolein where the more curved phase, QGII, was destabilized at higher pressure in favour of the less curved lamellar phase whilst increasing the temperature stabilized the more curved phases over the lamellar phase.7
H n shows an anomalous trend with increasing temperature, where H20 °Cn > H15 °Cn (Fig. 3a); this is attributed to the greater error in H15 °Cn compared to H20 °Cn (Fig. S4†) and is due to the accuracy of An and Vn obtained by model fitting. The curvature elastic energy of a mesophase is dependent on the accuracy of the pivotal surface parameters and thus the measured lattice parameters which are a reflection of changes to the molecular shape, therefore the curvature elastic energy is a subtle function of the molecular shape. In addition, experimental errors, from loss of water or lipid during sample preparation will lead to errors in the water volume fraction, consequently affecting the accuracy of An and Vn.
Conclusions
By fitting isothermal and isobaric swelling data with models which constrain the interfacial geometry of a lipid bilayer, we have shown for the first time the effect of pressure and temperature on the location of the pivotal surface and shown that the model fits the data consistently and provides a self-consistent explanation for the swelling behavior in the QGII phase. The lattice parameter of the unit cell is directly related to the molecular shape at a given pressure and temperature, consequently the pivotal surface parameters are a result of the lateral stress profiles at any pressure and temperature. Therefore pivotal surface parameters at certain pressures and temperatures give predictive power of the swelling behavior in the QGII phase with reasonable accuracy. Qualitative trends in the mean curvature as a function of water content, temperature and pressure are consistent with experimental phase behavior, affirming that within the precision of our measurements the geometric models replicate swelling behavior well. These observations reinforce the importance of these geometric models for describing curved bilayers and are a good basis for a full theoretical description of lyotropic phase behavior.Our experiments so far have been limited to the QGII phase, and further investigations on the pivotal surface parameters in the QDII and QPII phase are required for a full discussion on the accuracy of the geometric models for obtaining the pivotal surface parameters in the inverse bicontinuous cubic phases. Furthermore, in order to disentangle the contributing forces to the energetics of the inverse bicontinuous cubic phases we require a deeper understanding of the effect of changing the lateral stress profiles on the curvature elasticity.
Acknowledgements
This work was supported by EPSRC Platform Grant EP/G00465X and by an EPSRC DTA studentship awarded to T-YDT. We acknowledge Diamond Light Source (UK) and the European Synchrotron Radiation Facility (Grenoble, France) for the provision of synchrotron radiation facilities and we would like to thank Dr Claire Pizzey (beamline I22, DLS), and Dr Michael Sztucki (beamline ID02, ESRF) for their assistance during the synchrotron experiments.References
- A. H. Schoen, Report D-5541, NASA, Washington DC, 1970 Search PubMed.
- H. Schwarz, Gesammelte Mathematische Abhandlungen, Springer, Berlin, 1890 Search PubMed.
- D. F. Evans and H. Wennerstrom, The colloidal domain, where physics, chemistry, biology and technology meet, 1999 Search PubMed.
- G. L. Kirk, S. M. Gruner and D. L. Stein, Biochemistry, 1984, 23, 1093–1102 CrossRef CAS.
- E. S. Lutton, J. Am. Oil Chem. Soc., 1965, 42, 1068–1070 CrossRef CAS.
- M. Caffrey, J. Briggs and H. Chung, J. Phys. II, 1996, 6, 723–751 CrossRef.
- T. Y. D. Tang, N. J. Brooks, C. Jeworrek, O. Ces, N. J. Terrill, R. Winter, R. H. Templer and J. M. Seddon, Langmuir, 2012, 28, 13018–13024 CrossRef CAS PubMed.
- R. Winter, Biophys. J., 2002, 82, 153A Search PubMed.
- R. Winter, Curr. Opin. Colloid Interface Sci., 2001, 6, 303–312 CrossRef CAS.
- P. M. Duesing, J. M. Seddon, R. H. Templer and D. A. Mannock, Langmuir, 1997, 13, 2655–2664 CrossRef CAS.
- C. V. Kulkarni, T.-Y. Tang, A. M. Seddon, J. M. Seddon, O. Ces and R. H. Templer, Soft Matter, 2010, 6, 3191–3194 RSC.
- G. Cevc, A. Watts and D. Marsh, Biochemistry, 1981, 20, 4955–4965 CrossRef CAS.
- B. J. Boyd, D. V. Whittaker, S.-M. Khoo and G. Davey, Int. J. Pharm., 2006, 309, 218–226 CrossRef CAS PubMed.
- J. Bender, M. B. Ericson, N. Merclin, V. Iani, A. Rosen, S. Engstrom and J. Moan, J. Controlled Release, 2005, 106, 350–360 CrossRef CAS PubMed.
- G. O. Hustad, T. Richardson, W. C. Winder and M. P. Dean, Chem. Phys. Lipids, 1971, 7, 61–74 CrossRef CAS.
- N. J. Brooks, B. L. L. E. Gauthe, N. J. Terrill, S. E. Rogers, R. H. Templer, O. Ces and J. M. Seddon, Rev. Sci. Instrum., 2010, 81, 064103 CrossRef PubMed.
- R. G. Kimber, A. B. Walker, G. E. Schröder-Turk and D. J. Cleaver, Phys. Chem. Chem. Phys., 2010, 12, 844–851 RSC.
- V. Cherezov, J. Mol. Biol., 2006, 357, 1605–1618 CrossRef CAS PubMed.
- P. Nollert, H. Qiu, M. Caffrey, J. P. Rosenbusch and E. Landau, FEBS Lett., 2001, 179–186 CrossRef CAS.
- G. Rummel, A. Hardmeyer, C. Widmer, M. Chiu, P. Nollert, K. Locher, I. Pedruzzi, E. Landau and J. Rosenbusch, J. Struct. Biol., 1998, 182–191 Search PubMed.
- C. Leal, N. F. Bouxsein, K. K. Ewert and C. R. Safinya, J. Am. Chem. Soc., 2010, 132, 16841–16847 CrossRef CAS PubMed.
- R. H. Templer, J. M. Seddon and N. A. Warrender, Biophys. Chem., 1994, 49, 1–12 CrossRef CAS.
- R. H. Templer, Langmuir, 1995, 11, 334–340 CrossRef CAS.
- S. M. Gruner, J. Phys. Chem., 1989, 93, 7562–7570 CrossRef CAS.
- S. T. Hyde, J. Phys. Chem., 1989, 93, 1458–1464 CrossRef CAS.
- D. M. Anderson, S. M. Gruner and S. Leibler, Proc. Natl. Acad. Sci. U. S. A., 1988, 85, 5364–5368 CrossRef CAS.
- K. Grosse-Brauckmann, J. Colloid Interface Sci., 1997, 187, 418–428 CrossRef CAS.
- K. Grosse-Brauckmann, Interface Focus, 2012, 2, 582–588 CrossRef PubMed.
- P. Padmanabhan, F. J. Martinez-Veracoechea, J. C. Araque and F. A. Escobedo, J. Chem. Phys., 2012, 136 Search PubMed.
- C. Czeslik, R. Winter, G. Rapp and K. Bartels, Biophys. J., 1995, 68, 1423–1429 CrossRef CAS.
- O. Reis and R. Winter, Langmuir, 1998, 14, 2903–2909 CrossRef CAS.
- J. M. Seddon, A. M. Squires, C. E. Conn, O. Ces, A. J. Heron, X. Mulet, G. C. Shearman and R. H. Templer, Philos. Trans. R. Soc., A, 2006, 364, 2635–2655 CrossRef CAS PubMed.
- R. H. Templer, J. M. Seddon, P. M. Duesing, R. Winter and J. Erbes, J. Phys. Chem. B, 1998, 102, 7262–7271 CrossRef CAS.
- G. C. Shearman, O. Ces and R. H. Templer, Soft Matter, 2010, 6, 256–262 RSC.
- U. S. Schwarz and G. Gompper, Langmuir, 2001, 17, 2084–2096 CrossRef CAS.
- Y. Deng, Z. A. Almsherqi, M. M. L. Ng and S. D. Kohlwein, Trends Cell Biol., 2010, 20, 371–379 CrossRef CAS PubMed.
- T. Landh, FEBS Lett., 1995, 369, 13–17 CrossRef CAS.
- D. C. Turner, Z.-G. Wang, S. M. Gruner, D. A. Mannock and R. N. McElhaney, J. Phys. II, 1992, 2, 2039–2063 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: See DOI: 10.1039/c4sm00114a |
| This journal is © The Royal Society of Chemistry 2014 |