Counterion condensation on spheres in the salt-free limit†
Abstract
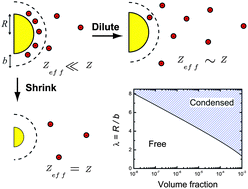
A highly-charged spherical colloid in a salt-free environment exerts such a powerful attraction on its counterions that a certain fraction condenses onto the surface of a particle. The degree of condensation depends on the curvature of the surface. So, for instance, condensation is triggered on a highly-charged sphere only if the radius exceeds a certain critical radius R*. R* is expected to be a simple function of the volume fraction of particles. To test these predictions, we prepare spherical particles which contain a covalently-bound ionic liquid, which is engineered to dissociate efficiently in a low-dielectric medium. By varying the proportion of ionic liquid to monomer we synthesise nonpolar dispersions of highly-charged spheres which contain essentially no free co-ions. The only ions in the system are counterions generated by the dissociation of surface-bound groups. We study the electrophoretic mobility of this salt-free system as a function of the colloid volume fraction, the particle radius, and the bare charge density and find evidence for extensive counterion condensation. At low electric fields, we observe excellent agreement with Poisson–Boltzmann predictions for counterion condensation on spheres. At high electric fields however, where ion advection is dominant, the electrophoretic mobility is enhanced significantly which we attribute to hydrodynamic stripping of the condensed layer of counterions from the surface of the particle.

 Please wait while we load your content...
Please wait while we load your content...