Hammett correlations as test of mechanism of CO-induced disulfide elimination from dinitrosyl iron complexes†
Abstract
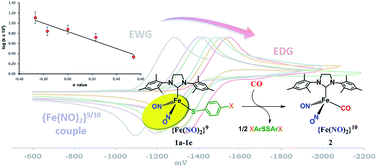
The displacement of RS˙ from [(NHC)(SPh)Fe(NO)2] (NHC = N-heterocyclic carbene) by carbon monoxide follows associative kinetics, rate = k [CO]1 [(NHC)(SPh)Fe(NO)2]1, resulting in reduction of the oxidized form of the dinitrosyliron unit, {Fe(NO)2}9 (Enemark–Feltham notation) to {Fe(NO)2}10. Thermodynamically driven by the release of PhS–SPh concomitant with formation of [(NHC)(CO)Fe(NO)2], computational studies suggested the reactant dinitrosyliron unit serves as a nucleophile in the initial slanted interaction of the π* orbital of CO, shifting into normal linear Fe–CO with weakening of the Fe–SPh bond. The current study seeks to experimentally test this proposal. A series of analogous {Fe(NO)2}9 [(NHC)(p-S–C6H4X)Fe(NO)2] complexes, with systematic variation of the para-substituents X from electron donor to electron withdrawing groups was used to monitor variation in electron density at the Fe(NO)2 unit via Hammett analyses. Despite the presence of non-innocent NO ligands, data from ν(NO) IR spectroscopy and cyclic voltammetry showed consistent tracking of the electron density at the {Fe(NO)2} unit in response to the aryl substituent. The electronic modifications resulted in systematic changes in reaction rates when each derivative was exposed to CO. A plot of the rate constants and the Hammett parameter σp is linear with a negative slope and a ρ value of −0.831; such correlation is indicative of rate retardation by electron-withdrawing substituents, and provides experimental support for the unique role of the delocalized frontier molecular orbitals of the Fe(NO)2 unit.

 Please wait while we load your content...
Please wait while we load your content...