Dissecting the reaction of Phase II metabolites of ibuprofen and other NSAIDS with human plasma protein†
Abstract
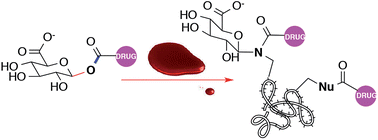
Nonsteroidal anti-inflammatory drugs (NSAIDs) are among the most widely used drugs on the market. Whilst they are considered safe, several NSAIDs have been withdrawn from the market as a result of adverse drug reactions. NSAIDs are extensively metabolised to their 1-β-O-acyl glucuronides (AGs), and the risk of NSAID AGs covalently modifying biomacromolecules such as proteins or DNA, leading to immune responses and cellular dysfunction constitutes a major concern in drug discovery and development. The assessment of the degree of protein modification and potential toxicity of individual NSAID AGs is therefore of importance in both drug monitoring and development. Herein, we report the covalent reaction of 1-β-O-acyl glucuronides of ibuprofen and several NSAID analogues with human serum albumin (HSA) protein in vitro under concentrations encountered in therapy. Stable transacylation and glycosylation adducts are formed; the observed protein product ratios can be rationalised by the degree of α-substitution in the acyl group. Structure-based protein reactivity correlations of AGs, such as these, may prove a useful tool in distinguishing between carboxylic acid-containing drugs of similar structure that ultimately prove beneficial (e.g., ibuprofen) from those that prove toxic (e.g., ibufenac).
- This article is part of the themed collection: ISACS16: Challenges in Chemical Biology

 Please wait while we load your content...
Please wait while we load your content...