Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Photocatalytic water oxidation at soft interfaces†
Malte
Hansen
a,
Fei
Li
*b,
Licheng
Sun
*bc and
Burkhard
König
*a
aInstitute of Organic Chemistry, University of Regensburg, D-93040 Regensburg, Germany. E-mail: burkhard.koenig@ur.de
bState Key Laboratory of Fine Chemicals, DUT-KTH Joint Education and Research Centre on Molecular Devices, Dalian University of Technology (DUT), Dalian 116012, PR China. E-mail: lifei@dlut.edu.cn; lichengs@kth.se
cDepartment of Chemistry, KTH Royal Institute of Technology, 10044 Stockholm, Sweden
First published on 1st May 2014
Abstract
Molecular water oxidation catalysts have been, for the first time, co-embedded with a photosensitizer into phospholipid membranes. The functionalized small unilamellar vesicles produce molecular oxygen by photocatalysis when irradiated with visible light in aqueous buffer. The two dimensional assembly of the catalysts at the lipid–water interface mimics photoactive membranes in biology and allows photocatalytic water oxidation at very low catalyst concentrations of 500 nM, which cannot be reached in homogeneous systems. Highest TONs are obtained below the membrane's main transition temperature indicating that phase separation, clustering and a limited dynamic enhance the photocatalytic activity of the assembly. The concept of membrane co-embedding can be applied to various combinations, ratios and concentrations of photosensitizers and water oxidizing catalysts, providing a new approach for artificial photosynthesis.
Introduction
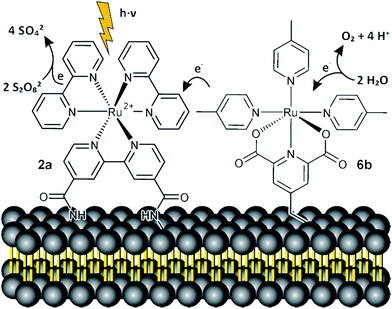
Efficient photochemical water splitting is still a scientific challenge.1,2 The overall process consists of an oxidative and a reductive half reaction with the water oxidation step involving a four electron transfer and highly reactive oxygen intermediates being considered as particularly difficult.3 Heterogeneous and homogeneous photocatalysts have been developed. A typical homogeneous catalyst for photooxidation of water consists of two subunits: A light absorbing photoredox active dye or sensitizer and the water oxidizing catalyst. The two subunits can be covalently connected (Fig. 1, 1, top), which ensures an efficient electron transfer, but requires the synthesis of complex ligands and linkers.4–8 If dye and oxidation catalyst are prepared as separate entities (Fig. 1, 4 and 5, bottom) different combinations can be easily realized, but the electron transfer between the subunits in homogeneous solution is diffusion controlled and depends on the concentration.9 In biological photosynthesis, chromophores and catalytic units are bound to membranes. Compared to homogeneous solution this two dimensional assembly increases the local concentration of the redox partners and shortens the average distance for electron transfer between them.10 Clustering of membrane embedded compounds and phase separation may increase this effect further. Compared to covalently connected sensitizer–catalyst pairs,11 the assembly in fluid membranes remains dynamic allowing continuous self-assembly and reorganization of the catalytic subunits, which may be advantageous for the catalytic performance. Following this biological model we have prepared phospholipid membranes with co-embedded amphiphilic photosensitizers and water oxidation catalysts.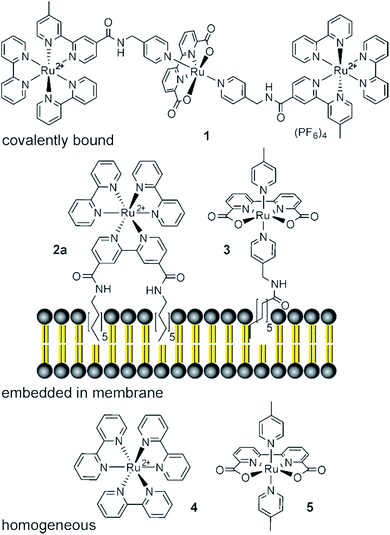 | ||
| Fig. 1 Interaction of photosensitizer–water oxidation catalysts, which are covalently connected (top),8 membrane co-embedded (middle) or in homogeneous solution (bottom).12 | ||
Such functionalized vesicles place the catalytic subunits in close proximity even at very low overall concentrations while still allowing the easy variation of sensitizer–catalyst combinations, ratios and concentrations.
Results and discussion
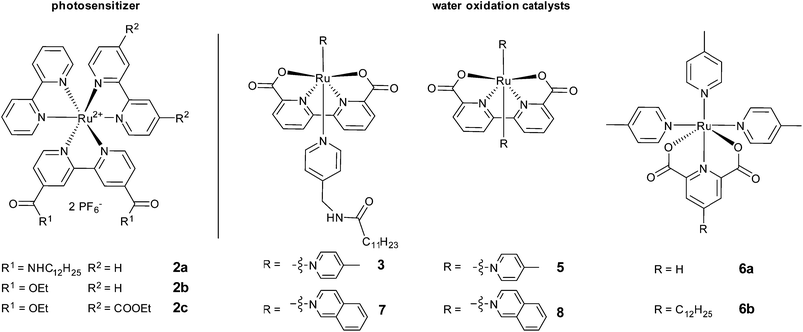
For anchoring into a self-assembled membrane the photosensitizer and the water oxidation catalyst should be amphiphilic and bear a hydrophobic alkyl chain. Derivative 2a of the widely used photosensitizer ruthenium tris-bipyridine (4) is suitable for membrane embedding and was prepared according to reported procedures.13–17 Three amphiphilic water oxidation catalysts 3, 6b and 7 (Fig. 2) were derived from literature known catalysts18–20 that have shown good performance in chemical and photochemical water oxidation in homogeneous solution. Details of their synthesis and characterization are given in the supplementary information.Vesicles were prepared by sonication of phospholipid films with added photosensitizer 2a (12.5 mol%) and water oxidation catalysts 6b, 3 or 7 (0.05–1.25 mol%) in phosphate buffer (50 mM, pH = 7.0) containing the sacrificial electron acceptor sodium persulfate. Dynamic light scattering confirmed a narrow size distribution of the vesicles in all cases and UV spectra of the solutions showed absorption maxima and extinction coefficients of the photosensitizer comparable to a homogeneous solution. The influence of vesicle size and solution turbidity on oxygen evolution was negligible (for data see ESI†).
Degassed solutions of the functionalized vesicles containing photosensitizer 2a and water oxidation catalyst 6b were irradiated with high power LEDs (λ = 455 nm; 200 mW cm−2 light intensity) and the oxygen evolution was monitored by an optical probe and quantified by head space gas chromatography. The experimental details and data are given in the ESI.†Fig. 3 shows the proposed reaction mechanism.
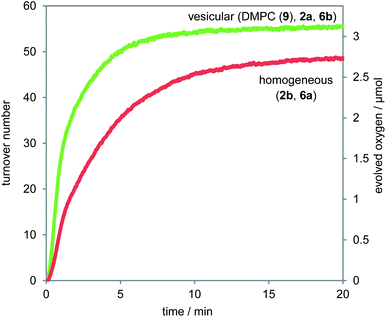
To investigate the effect of the membrane embedding on the efficiency of the photocatalytic water oxidation, the oxygen evolution of a homogeneous aqueous solution containing photosensitizer 2b (125 μM) and water oxidation catalyst 6a (12.5 μM)18 was compared under otherwise identical conditions with a vesicular DMPC (1,2-dimyristoyl-sn-glycero-3-phosphocholine, 9, Fig. 5) solution containing photosensitizer 2a (125 μM) and water oxidation catalyst 6b (12.5 μM). The observed turnover numbers (TON) of 49 for the homogeneous and 55 for the vesicular solution are comparable ( Fig. 4, Table 1).
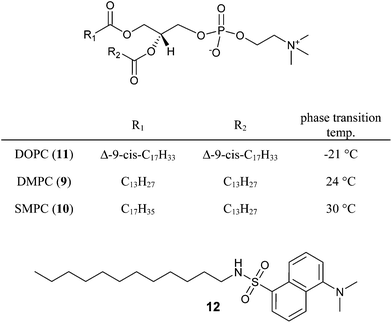 | ||
| Fig. 5 Structures and phase transition temperatures of the phospholipids DMPC (9), SMPC (10) and DOPC (11); solvatochromic dye 12. | ||
| Entry | cat | PS | Ratio cat![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PS PS |
c cat/μM | c DMPC/μM | n (O2)/μmol | TON |
|---|---|---|---|---|---|---|---|
| a Control experiments without catalyst 6b (entry 9) and photosensitizer 2b (entry 10), respectively, resulted in no detectable oxygen formation. | |||||||
| 1 | 6b | 2a | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
12.5 | 863 | 3.1 | 55 |
| 2 | 6a | 2b | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
12.5 | — | 2.8 | 49 |
| 3 | 6b | 2a | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
6.25 | 869 | 2.1 | 75 |
| 4 | 6a | 2b | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
6.25 | — | 1.0 | 37 |
| 5 | 6b | 2a | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
2.5 | 873 | 2.1 | 192 |
| 6 | 6a | 2b | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
2.5 | — | 0.2 | 22 |
| 7 | 6b | 2a | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250 |
0.5 | 873 | 0.6 | 394 |
| 8 | 6a | 2b | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250 |
0.5 | — | 0 | 0 |
| 9 | — | 2a | — | — | 875 | 0 | 0 |
| 10 | 6b | — | — | 12.5 | 988 | 0 | 0 |
Next, in a series of experiments the amount of the water oxidizing catalyst 6 was reduced and the performances of the homogeneous and the vesicular system were compared. A concentration of 6.25 μM of complex 6a in homogeneous solution or complex 6b in vesicular solution corresponds to a catalyst to sensitizer ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 and gave TONs of oxygen evolution of 37 (Table 1, entry 4) and 75 (entry 3), respectively. At a ratio of 1
20 and gave TONs of oxygen evolution of 37 (Table 1, entry 4) and 75 (entry 3), respectively. At a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 using a concentration of the catalysts 6 of 2.5 μM in the presence of 125 μM of the photosensitizers 2 the difference in TONs in homogeneous solution of 22 (entry 6) and the vesicular solution of 192 (entry 5) were even more pronounced (Table 1). At 500 nM concentration of catalyst 6 water photooxidation is negligible in homogeneous solution, while in the membrane a TON of 394 is observed (entry 7).
50 using a concentration of the catalysts 6 of 2.5 μM in the presence of 125 μM of the photosensitizers 2 the difference in TONs in homogeneous solution of 22 (entry 6) and the vesicular solution of 192 (entry 5) were even more pronounced (Table 1). At 500 nM concentration of catalyst 6 water photooxidation is negligible in homogeneous solution, while in the membrane a TON of 394 is observed (entry 7).
The results demonstrate one advantage of the co-embedding of photosensitizer and water oxidation catalyst in a vesicle membrane: the two dimensional arrangement at the lipid–water interface places the subunits of the catalyst even at low overall concentrations in close proximity, which favors the electron transfer and their concerted action in the water oxidation. Photocatalytically active sensitizer catalyst combinations are possible in the vesicular system at ratios and concentrations, which cannot be realized in homogeneous solution.
In previous studies, the non-amphiphilic derivatives 5 and 8 of ruthenium complexes 3 and 7 have shown a better water oxidation performance compared to complex 6 in homogeneous solution.12,19 To prove the versatility of membrane co-embedding for the preparation of water photooxidation assemblies, catalysts 3 or 7 (2.5 μM) and photosensitizer 2a (125 μM) were added to DMPC (9) vesicles. Vesicular and homogeneous samples were irradiated with a 500 W xenon lamp (cut off filter λ > 400 nm; 450 mW cm−2 light intensity) and the amount of evolved oxygen was determined (Table 2). The catalyst to sensitizer ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 gave TONs of 157 for catalyst 3 (entry 2) and of 185 for catalyst 7 (entry 3), which are higher compared to the TON of 109 obtained in homogeneous solutions (entry 1) using the non-amphiphilic derivative of 3 bearing 2 axial 4-methylpyridine ligands (5)12 (Fig. 2) and the photosensitizer 2a, but otherwise identical conditions.
50 gave TONs of 157 for catalyst 3 (entry 2) and of 185 for catalyst 7 (entry 3), which are higher compared to the TON of 109 obtained in homogeneous solutions (entry 1) using the non-amphiphilic derivative of 3 bearing 2 axial 4-methylpyridine ligands (5)12 (Fig. 2) and the photosensitizer 2a, but otherwise identical conditions.
The performance of the water photooxidizing system is known to be limited by the stability of the photosensitizer,19 which applies also for the vesicular assemblies. By embedding of new photosensitizer into the membrane of used, irradiated vesicles they regain about 60% of their initial TON (entry 4) when irradiated again after removal of oxygen from the sample (see ESI† for data).
The physical properties of phospholipid membranes change significantly with the nature of the lipid. To investigate the effect on the water photooxidation the activity of embedded photosensitizer 2a (125 μM) and complex 6b (12.5 μM) were investigated in three membranes prepared from DMPC (9), SMPC (1-stearoyl-2-myristoyl-sn-glycero-3-phosphocholine) (10) and DOPC (1,2-dioleoyl-sn-glycero-3-phosphocholine) (11). The three lipids have the same dipolar head group and differ only in the length and structure of the hydrocarbon chains (Fig. 5). The polarity at the lipid–water interface is therefore expected to be very similar, which was confirmed by the embedded amphiphilic solvatochromic dansyl dye 12 showing the same optical properties in all three membranes (see ESI†). However, in DMPC (9) and SMPC (10) (Table 3) the co-embedded complexes produced oxygen upon irradiation (λ = 455 nm; 200 mW cm−2 light intensity), while the activity in DOPC (11) (entry 1) was significantly lower. We explain the effect by the distinct differences in membrane fluidity. DMPC (9) and SMPC (10) have main phase transition temperatures from the gel to the liquid crystalline phase of 24 °C and 30 °C,21 but DOPC (11) with a transition temperature of −21 °C is already in the liquid crystalline phase at the temperature of the experiment. Photooxidation experiments of DMPC (9) and SMPC (10) vesicles with embedded photosensitizers and catalyst at temperatures above their transient temperatures (entries 5 and 7) showed a reduced activity, while the activity remains unchanged at temperatures below the phase transition (entries 4 and 6). We conclude that phase separation and clustering of the embedded complexes, expected for the amphiphilic additives 2a and 6b below the transition temperature of a lipid membrane, enhances the photocatalytic activity of the assembly.22 This is supported by the difference in quantum efficiency φ = (1 − I/I0) determined from the fluorescence quenching of 2a with sodium persulfate under the reaction conditions.23 While DMPC (9) and SMPC (10) vesicles show a quantum efficiency of φ = 35% and 30%, respectively, a significantly lower value of φ = 10% was determined for DOPC (11) vesicles (see ESI† for data).
| Entry | Phospholipid | T/°C | n (O2)/μmol | TON |
|---|---|---|---|---|
| 1 | DOPC (11) | 25 | 0.2 | 3 ± 1 |
| 2 | DMPC (9) | 25 | 3.1 | 55 ± 2 |
| 3 | SMPC (10) | 25 | 3.6 | 64 ± 4 |
| 4 | DMPC (9) | 14 | 3.2 | 66 ± 4 |
| 5 | DMPC (9) | 34 | 2.4 | 52 ± 3 |
| 6 | SMPC (10) | 20 | 3.6 | 65 ± 2 |
| 7 | SMPC (10) | 40 | 1.7 | 29 ± 6 |
Conclusion
In summary, we have, for the first time, self-assembled photosensitizer–catalyst water oxidation systems by co-embedding of two amphiphilic ruthenium complexes into the phospholipid bilayer membrane of small unilamellar vesicles. The observed oxygen production upon light irradiation is comparable to similar systems in homogeneous solutions, but superior at low concentrations of the water oxidation catalysts. Membrane embedded water photooxidation systems remain catalytically active at concentrations, where homogeneous mixtures of photosensitizer and water oxidation catalyst are inoperable. Phase separation and patch formation cluster the complexes in the membrane, which might facilitate the intermolecular electron transfer processes. The fluidity of the membrane affects the self-organization of the embedded complexes and therefore their photocatalytic performance. Highest TONs are observed in gel phase membranes, where phase separation is favored. The method was applied to different combinations of sensitizers and oxidation catalysts and allows a rapid screening of sensitizer–catalyst combinations, ratios and concentration ranges. Functionalized vesicles may be transferred and spread onto a variety of surfaces, which may allow the processing, immobilization or printing of photocatalytically active membranes for further applications in functional devices for artificial photosynthesis.Acknowledgements
This work was supported by the German Science Foundation (DFG) and the National Natural Science Foundation of China (21361130020 and 21120102036) and National Basic Research Program of China (973 program, 2014CB239402).Notes and references
- T. R. Cook, D. K. Dogutan, S. Y. Reece, Y. Surendranath, T. S. Teets and D. G. Nocera, Chem. Rev., 2010, 110, 6474–6502 CrossRef CAS PubMed.
- N. S. Lewis and D. G. Nocera, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 15729–15735 CrossRef CAS PubMed.
- M. Hambourger, G. F. Moore, D. M. Kramer, D. Gust, A. L. Moore and T. A. Moore, Chem. Soc. Rev., 2008, 38, 25–35 RSC.
- P. D. Frischmann, K. Mahata and F. Wurthner, Chem. Soc. Rev., 2013, 42, 1847–1870 RSC.
- M. R. Norris, J. J. Concepcion, D. P. Harrison, R. A. Binstead, D. L. Ashford, Z. Fang, J. L. Templeton and T. J. Meyer, J. Am. Chem. Soc., 2013, 135, 2080–2083 CrossRef CAS PubMed.
- N. Kaveevivitchai, R. Chitta, R. Zong, M. El Ojaimi and R. P. Thummel, J. Am. Chem. Soc., 2012, 134, 10721–10724 CrossRef CAS PubMed.
- L. Kohler, N. Kaveevivitchai, R. Zong and R. P. Thummel, Inorg. Chem., 2013, 53, 912–921 CrossRef PubMed.
- F. Li, Y. Jiang, B. Zhang, F. Huang, Y. Gao and L. Sun, Angew. Chem., Int. Ed., 2012, 51, 2417–2420 CrossRef CAS PubMed.
- D. J. Wasylenko, R. D. Palmer and C. P. Berlinguette, Chem. Commun., 2013, 49, 218–227 RSC.
- Electron transfer in membranes was intensively investigated. For selected references, see: (a) S. Bhosale, A. L. Sisson, P. Talukdar, A. Fürstenberg, N. Banerji, E. Vauthey, G. Bollot, J. Mareda, C. Röger, F. Würthner, N. Sakai and S. Matile, Science, 2006, 313, 84–86 CrossRef CAS PubMed; (b) J. J. Grimaldi, S. Boileau and J.-M. Lehn, Nature, 1977, 265, 229–230 CrossRef CAS; (c) R. F. Khairutdinov and J. K. Hurst, Nature, 1999, 402, 509–511 CrossRef CAS; (d) A. Perez-Velasco, V. Gorteau and S. Matile, Angew. Chem., Int. Ed., 2008, 47, 921–923 CrossRef CAS PubMed; (e) G. Steinberg-Yfrach, P. A. Liddell, S.-C. Hung, A. L. Moore, D. Gust and T. A. Moore, Nature, 1997, 385, 239–241 CrossRef CAS; (f) L. Zhu, R. F. Khairutdinov, J. L. Cape and J. K. Hurst, J. Am. Chem. Soc., 2005, 128, 825–835 CrossRef PubMed.
- H. Dau, C. Limberg, T. Reier, M. Risch, S. Roggan and P. Strasser, ChemCatChem, 2010, 2, 724–761 CrossRef CAS.
- L. Duan, Y. Xu, P. Zhang, M. Wang and L. Sun, Inorg. Chem., 2009, 49, 209–215 CrossRef PubMed.
- W. S. Aldridge, B. J. Hornstein, S. Serron, D. M. Dattelbaum, J. R. Schoonover and T. J. Meyer, J. Org. Chem., 2006, 71, 5186–5190 CrossRef CAS PubMed.
- V. Aranyos, A. Hagfeldt, H. Grennberg and E. Figgemeier, Polyhedron, 2004, 23, 589–598 CrossRef CAS PubMed.
- S. Füldner, R. Mild, H. I. Siegmund, J. A. Schroeder, M. Gruber and B. König, Green Chem., 2010, 12 Search PubMed.
- D. Pucci, G. Barberio, A. Bellusci, A. Crispini, M. Ghedini and E. I. Szerb, Mol. Cryst. Liq. Cryst., 2005, 441 Search PubMed.
- M. S. Vickers, K. S. Martindale and P. D. Beer, J. Mater. Chem., 2005, 15 Search PubMed.
- L. Duan, Y. Xu, M. Gorlov, L. Tong, S. Andersson and L. Sun, Chem.– Eur. J., 2010, 16, 4659–4668 CrossRef CAS PubMed.
- L. Wang, L. Duan, L. Tong and L. Sun, J. Catal., 2013, 306, 129–132 CrossRef CAS PubMed.
- L. Duan, C. M. Araujo, M. S. G. Ahlquist and L. Sun, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 15584–15588 CrossRef CAS PubMed.
- J. R. Silvius, Lipid-Protein Interactions, John Wiley & Sons, Inc, New York, 1982 Search PubMed.
- The varying fluorescence intensity observed in different lipid vesicular solutions without persulfate indicates changes in the local chemical environment. These may also be influenced by parameters like rigidity of the membrane, solvation of the artificial amphiphiles or changing positions within the bilayer.
- A. Lewandowska-Andralojc and D. E. Polyansky, J. Phys. Chem. A, 2013, 117, 10311–10319 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4sc01018c |
| This journal is © The Royal Society of Chemistry 2014 |