Generation of gold carbenes in water: efficient intermolecular trapping of the α-oxo gold carbenoids by indoles and anilines†
Abstract
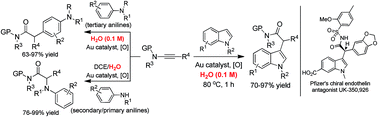
The efficient intermolecular reaction of gold carbene intermediates, generated via gold-catalyzed alkyne oxidation, with indoles and anilines has been realized in aqueous media. Importantly, it was revealed for the first time that water could dramatically suppress the undesired over-oxidation, providing a general and practical solution to the problem of over-oxidation in gold-catalyzed intermolecular alkyne oxidation with external nucleophiles. This strategy was successfully applied to the formal synthesis of the Pfizer's chiral endothelin antagonist UK-350,926.

 Please wait while we load your content...
Please wait while we load your content...