The proton conduction mechanism in a material consisting of packed acids†
Abstract
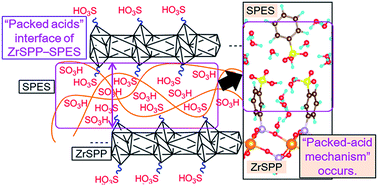
Proton conduction due to acid–acid interactions is an important topic in a variety of fields, from materials science to biochemistry. We observed a distinctive proton conduction phenomenon for a material consisting of packed acids at the interface of zirconium sulphophenylphosphonate (ZrSPP) and sulphonated poly(arylene ether sulphone) (SPES). The proton in the composite was found to be active, while water, a general proton carrier, remained immobile. Moreover, the conductivity of the composite material was higher than the sum of the individual conductivities of ZrSPP and SPES, which can be attributed to the packed acids present at the interface. We propose a “packed-acid mechanism” based on the results of ab initio calculations in order to explain such a significant and interesting behaviour of protons. During common proton conduction, pseudo-shuttling of a proton between a proton donor and acceptor is a general event that disrupts the reorientation phenomenon, which is an important process associated with common proton conduction. Based on our results, it could be inferred that in packed acid materials, the acid–acid interaction eliminates the pseudo-shuttling (interception) and facilitates reorientation, resulting in successive proton conduction.

 Please wait while we load your content...
Please wait while we load your content...