Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The ionic liquid–vacuum outer atomic surface: a low-energy ion scattering study†
Ignacio J.
Villar-Garcia
a,
Sarah
Fearn
a,
Gilbert F.
De Gregorio
b,
Nur L.
Ismail
b,
Florence J. V.
Gschwend
c,
Alastair J. S.
McIntosh
b and
Kevin R. J.
Lovelock
*b
aDepartment of Materials, Imperial College London, Exhibition Road, South Kensington, SW7 2AZ, UK. E-mail: kevin.lovelock@imperial.ac.uk; Tel: +44 (0)20 7594 5868
bDepartment of Chemistry, Imperial College London, Exhibition Road, South Kensington, SW7 2AZ, UK
cDepartment of Chemical Engineering, Imperial College London, Exhibition Road, South Kensington, SW7 2AZ, UK
First published on 8th July 2014
Abstract
We have identified elements present in the ionic liquid–vacuum outer atomic surface of 23 ionic liquids using high sensitivity low-energy ion scattering (LEIS), a very surface sensitive technique. We show that the probability of cationic heteroatoms being present at the ionic liquid–vacuum outer atomic surface is very low; we detected imidazolium nitrogen for only one of the 18 imidazolium based ionic liquids investigated, no nitrogen for the two ammonium based ionic liquids and a very small amount of phosphorus for two of the three phosphonium-based ionic liquids. We determine that the anion is always present at the ionic liquid–vacuum outer atomic surface, even for very large cations containing dodecyl alkyl chains or longer; these chains dominate the ionic liquid–vacuum outer atomic surface, but are not sufficiently densely packed to completely cover the anions. We demonstrate the presence of strong hydrogen bond acceptor adsorption sites at the ionic liquid–vacuum outer atomic surface. We demonstrate that the amount of ion present at the ionic liquid–vacuum outer atomic surface can be tuned by varying the size of the other ion; larger cations (or anions) occupy more of the ionic liquid–vacuum outer atomic surface, leaving less room for anions (or cations). By identifying elements present at the ionic liquid–vacuum outer atomic surface, conclusions can be drawn on the orientations of anions nearest the vacuum. We show that for five different anions there is a most probable ion orientation, but other anion orientations also exist, demonstrating the presence of multiple anion orientations. The imidazolium cations nearest to the vacuum also show similar multi-orientation behaviour. This variety of atoms present and therefore ion orientations is expected to be central to controlling surface reactivity. In addition, our results can be used to quantitatively validate simulations of the ionic liquid–vacuum surface at a molecular level. Overall, our studies, in combination with literature data from different techniques and simulations, provide a clear picture of ionic liquid–vacuum outer atomic surfaces.
1. Introduction
The ionic liquid–gas surface1,2 is crucial for a wide range of applications: gas capture/storage/separation,3–9 nanoparticle and thin film preparation,10–14 supported ionic liquid phase (SILP) catalysis,15,16 and stationary phases for gas chromatography.17,18 For absorption to occur, which is required for all of the above applications, adsorption must first occur. Adsorption is primarily controlled by the composition of the ionic liquid–gas outer atomic surface (the atomic layer of an ionic liquid which is in contact with the gas). If the possible adsorption sites at the ionic liquid–gas outer atomic surface are known, then adsorption can be understood. In addition, the ionic liquid–gas surface is a key factor in determining the surface tension of ionic liquids.19,20A huge range of ionic liquids can be conceived due to the ability to vary the substituents on the cation and anion (a conservative estimate gives 106 ionic liquids made up of one cation and one anion21). Such an array of ionic liquids gives the tantalising possibility of tuning the ionic liquid–vacuum surface by varying substituents to give exactly the desired structure/properties.22 A brute force method of synthesising and measuring a large, structurally diverse set of ionic liquids is expensive and time-consuming. Consequently, there is a pressing requirement for greater understanding of the ionic liquid–vacuum surface to realise the goal of tunability. The only way this requirement can be achieved is by investigating carefully selected “target” ionic liquids that will give a representative picture for all ionic liquids and allow predictions of properties.
Investigating most liquid–gas surfaces at a molecular level is very difficult due to a lack of available techniques; many molecular level techniques require ultrahigh vacuum (UHV) conditions, which at room temperature lead to evaporation of most liquids.23 Consequently a limited range of methods have been used, including indirect probes (e.g. surface tension) and optical techniques (e.g. sum frequency generation (SFG) spectroscopy).24 Ionic liquids have sufficiently low vapour pressure that they can be studied at room temperature using standard UHV apparatus, opening up the possibility of using a wide range of surface science techniques to probe the molecular level structure of these liquid systems.13,25–27
The techniques that have been used to investigate the ionic liquid–vacuum surface (the region within which the properties of the ionic liquid are significantly different from that of the bulk ionic liquid) can be separated into four different areas: spectroscopy,28–45 scattering,46–59 sticking probability/temperature programmed desorption,60–62 and molecular dynamics (MD) simulations.45,63–78 These techniques probe different depths; the ionic liquid–vacuum experimental surface is that portion of the ionic liquid with which there is significant interaction with the particles/radiation used for excitation (the volume corresponding to the escape for the emitted radiation or particle). For example, the ionic liquid–vacuum experimental surface for angle-resolved X-ray photoelectron spectroscopy (ARXPS) is about 1 nm to 1.5 nm.28–33 However, metastable atom electron spectroscopy (MAES)35–39 and atom/molecule scattering,46–51 due to the reactivity/size of the probe, are significantly more surface sensitive than ARXPS,28–33 as these techniques probe primarily the ionic liquid–vacuum outer atomic surface (the atomic layer of an ionic liquid which is in contact with the vacuum). Different surface sensitive techniques offer different information, and to fully understand the ionic liquid–vacuum surface, a range of techniques are needed in order to build up a complete picture. Given the complexity of ionic liquid–vacuum surfaces, MD simulations have a vital role to play in gaining a clearer understanding. To date, MD simulations have been quantitatively validated by comparing experimental and calculated values of surface tension45,64–66 and CO2 scattering;70 molecular level structural validation is required to give confidence in the force fields used.
The most frequently postulated model, and therefore we conclude is the most probable model, for the ionic liquid–vacuum surface, typically based upon studies on 1,3-dialkylimidazolium-based ionic liquids, is that the alkyl carbons are nearest to the vacuum, and that the charged components are nearest to the bulk liquid.1,2,28–39,45–48,52–58,63–78 This model is the most probable, but is it truly representative of the complex ionic liquid–vacuum surface? In addition, will this model be sufficient to explain the surface properties of ionic liquids? In particular, are all adsorption sites accounted for? Using sticking probability and temperature programmed desorption for water adsorption/desorption from [C8C1Im][BF4] and [C2C1Im][Tf2N] ([CnC1Im]+ = 1-alkyl-3-methylimidazolium, [BF4]− = tetrafluoroborate and [Tf2N]− = bis[(trifluoromethane)sulfonyl]imide), Jones and co-workers showed that the thermally averaged kinetic parameters are very similar for these two very different ionic liquids.60–62 The findings are not easily explained using the most probable model; therefore, a more comprehensive description of ionic liquid–vacuum surfaces is required. In order to achieve this description, two key questions need to be answered. Firstly, are the cation and anion both at the ionic liquid–vacuum outer atomic surface? And secondly, which moieties of the cation and anion are at the ionic liquid–vacuum outer atomic surface? Answering the second question will allow conclusions to be drawn on ion orientation.
It is clear from literature reports that at least one moiety of cations is at the ionic liquid–vacuum outer atomic surface: the alkyl chains. It should be noted that the alkyl chains are not densely packed, as they have a density that is lower than close packing, as demonstrated using SFG spectroscopy43 and MD simulations.65,66 We cannot find evidence in the literature from an experimental perspective confirming charged cationic groups are at the ionic liquid–vacuum outer atomic surface. However, MD simulations have found imidazolium rings for [C2C1Im][BF4], [C4C1Im][PF6] ([PF6]− = hexafluorophosphate), [C6C1Im][Tf2N] and [C8C1Im][BF4] at the ionic liquid–vacuum outer atomic surface.63,65,66,71,72 It is envisaged that the imidazolium ring orientation will impact upon adsorption behaviour, as the adsorption site will be very different if the π-system of the imidazolium ring is oriented parallel or perpendicular to the surface plane. There is contradictory evidence in the literature about the imidazolium ring orientation for the ions nearest the vacuum. SFG spectroscopy studies on an extensive range of ionic liquids by Baldelli and co-workers have concluded that the imidazolium ring plane is oriented parallel to the ionic liquid–vacuum surface plane.1,41,42 Reports from MD simulations have concluded that the most probable imidazolium ring orientation is parallel66,67,70 or perpendicular64–66,71–74,77,78 to the ionic liquid–vacuum surface plane; it is unclear at this stage if the different orientations reported are due simulation effects or ionic liquids.
There is a consensus that at least part of the anion is at the ionic liquid–vacuum outer atomic surface when the cation contains short alkyl chains (n ≤ 4);1,2 however, it must be noted that using MD simulations it was concluded that even for [C4C1Im][BF4] there is relatively little anion at the ionic liquid–vacuum outer atomic surface.70 There is agreement between experiments29,35,46–48,52,59 and MD simulations48,65,70,77 that for [CnC1Im][Tf2N] the [Tf2N]− anion is at the ionic liquid–vacuum outer atomic surface, even when n = 10 and 12; however, it has also been concluded that [Tf2N]− is not at the ionic liquid–vacuum outer atomic surface for [C8C1Im][Tf2N].53 For n > 4, with anions other than [Tf2N]−, there is contradictory evidence in the literature. The conclusion from seven experimental studies on ionic liquids containing three different anions (Cl−, [BF4]−, [CF3SO3]− = trifluoromethanesulfonate, [Tf2N]−) using two different techniques, MAES and neutral impact collision ion scattering spectroscopy (NICISS), is that the anion is not located at the ionic liquid–vacuum outer atomic surface.35,36,53–57 In addition, coarse grain MD simulations on [CnC1Im][NO3] (n = 8 and 12, [NO3]− = nitrate) suggested that the [NO3]− anion is not at the ionic liquid–vacuum outer atomic surface.76 In contrast, Pensado et al. used MD simulations to conclude that for [C8C1Im][BF4] the anion is indeed located at the ionic liquid–vacuum outer atomic surface.66 Unlike for cations, there is no contradictory evidence in the literature about the anion orientation for the ions nearest the vacuum. The anion orientation reported is the most probable: for [Tf2N]− the CF3 groups are near the vacuum and the (SO2)2N groups are towards the bulk,29,52,65,68,70,73,77 for [CnOSO3]− the alkyl groups are near the vacuum and the OSO3 groups are towards the bulk ([CnOSO3]− = alkylsulfate),29,30,67 and for [CF3SO3]− the CF3 groups are near the vacuum and the SO3 groups are towards the bulk.34 Experimental reports on the possibility of more than one anion orientation are limited. Using RBS for [CnC1Im][Tf2N] it was concluded that for both n = 2 and n = 6, the most probable orientation is with the CF3 groups towards the vacuum; however, when n = 6 there is a small but significant probability of SO2N groups being towards the vacuum.52 Using SFG spectroscopy a range of [N(CN)2]− orientations were found in [C4C1Im][N(CN)2] ([N(CN)2]− = dicyanamide).40
Another important question arises: is it possible to control and therefore tune the amount of a particular ion at the outer surface, and therefore tune the surface properties? The effect of the anion on the amount of the cation (and vice versa) at the outer atomic surface has been investigated relatively little to date for ionic liquids, due most likely to a combination of small sample sets and the technique not being able to provide such information. Using ARXPS it was found for nine [C8C1Im][A] (with a wide range of [A]−) that an enrichment of the cation alkyl chains is found at the expense of charged cation groups and anions at a depth of 1 nm to 1.5 nm.31 This alkyl enhancement effect decreases with increasing size of the anion. It was concluded that smaller anions allow more cation (and therefore more alkyl) towards vacuum. Scattering of NO from [C4C1Im][A] ([A]− = Cl−, [BF4]− and [Tf2N]−) has led to the conclusion that anion size might be a key factor in determining surface properties.51
Low-energy ion scattering (LEIS) is one of the most surface sensitive techniques, as it allows identification of elements (apart from hydrogen and helium) present at the sample outer atomic surface. He+ ions are used as the probe and He+ ions are detected after being scattered from the sample surface. The neutralisation probability to form He0 after a He+-sample collision is very large.79,80 He+ ions that penetrate beyond the outer atomic surface will undergo multiple collisions. Therefore, LEIS is primarily sensitive to the sample outer atomic surface; the ionic liquid–vacuum experimental surface and the ionic liquid–vacuum outer atomic surface are effectively the same. Each element produces a signal that is composed of the characteristic surface peak due to atoms in the sample outer atomic surface. The energy of this peak is characteristic of each element, making element identification straightforward and unambiguous; the peak area can be related to the concentration of the element present at the sample outer atomic surface. The reactive scattering techniques used by the groups of McKendrick and Minton detect the presence of hydrogen at the ionic liquid–vacuum outer atomic surface.46–48 Therefore, LEIS and these reactive scattering techniques are complementary, as all elements apart from hydrogen and helium can be detected using LEIS.
LEIS has mostly been used to investigate metals and metal oxides,79,80 but has also been used twice to investigate ionic liquids.59,81 Caporali et al. used LEIS to investigate [C4C1Im][Tf2N]; they only observed a peak due to fluorine and no other peaks, and so concluded that the ionic liquid–vacuum outer atomic surface was primarily composed of fluorine.59 However, this LEIS data was not collected with a high-sensitivity LEIS spectrometer. Kauling et al. used high-sensitivity LEIS to investigate the distribution of gold nanoparticles in [C4C1Im][PF6] and four functionalised ionic liquids.81 After analysis of the neat ionic liquid spectra they concluded that the ionic liquid–vacuum outer atomic surface is composed of all atoms of both cations and anions for all ionic liquids studied. However, careful examination of the data in this paper reveals the absence of nitrogen peaks in those spectra.81
In this paper we use high-sensitivity LEIS to determine the elements present at the ionic liquid–vacuum outer atomic surface for 23 ionic liquids (see Table S1† for a list of the 23 ionic liquids with their full names). We have studied 18 dialkylimidazolium-based ionic liquids, two tetraalkylammonium-based ionic liquids, and three tetraalkylphosphonium-based ionic liquids, in combination with 12 different anions, which in total are composed of eight different non-hydrogen elements: B, C, N, O, F, P, S and Cl (see Scheme 1 for the cations and anions studied). We have selected our “target” ionic liquids with the goal of giving a representative picture for all ionic liquids and allowing predictions of properties. The ionic liquids we have studied range from those of mainly an academic interest (e.g. [Tf2N]− and [BF4]−) to those of an industrial interest (e.g. [C0OSO3]− and [C1CO2]− = acetate). We have chosen cations that best represent those studied/used, and have focused on dialkylimidazolium-based ionic liquids, as these are by far the most widely studied. Our anions span a wide range of shapes, sizes and properties, from the small Cl− to the large [Tf2N]−. In particular, there is a great deal of interest in ionic liquids composed of [C1CO2]− and [C0OSO3]− for catalysis,82 biomass dissolution,83 and CO2 capture.84 In addition, ionic liquids containing the [C0OSO3]− anion are amongst the cheapest available,85 and so their use industrially is expected to greatly increase in the coming years. The ionic liquid–vacuum surface is studied for the first time for a number of the ionic liquids here. We investigated whether the cation charged group and the anion are present at the ionic liquid–vacuum outer atomic surface. We investigate whether the amount of anion (or cation) can be tuned by varying the cation (or anion), and what anionic (or cationic) properties control this tuning. The orientation of the imidazolium cation and the orientation of five anions are investigated. In combination with results from other techniques and simulations, we will provide a clear picture of the ionic liquid–vacuum outer atomic surface for the ionic liquids investigated.
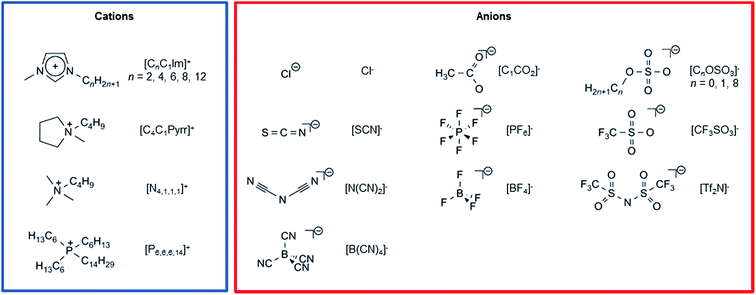 | ||
| Scheme 1 Cations and anions studied in this paper. A list of the 23 ionic liquids studied, with their full names, is given in Table S1.† | ||
2. Experimental
2.1. Ionic liquid synthesis
2.2. Low-energy ion scattering (LEIS)
LEIS experiments were performed in a Qtac 100 instrument (ION-TOF GmbH) at a base pressure of ∼3 × 10−10 mbar (which increases to the 10−8 mbar range during the analysis due to the flux of helium gas). The instrument is fitted with a double toroidal energy analyser (DTA) which collects the scattered ions at a scattering angle of 145° from all azimuth angles. This large solid angle of acceptance combined with parallel energy detection improves the efficiency of the detector by several orders of magnitude in comparison to conventional LEIS instruments and allows the acquisition of higher quality spectra for the same He+ doses. This is especially useful when static conditions are required, i.e. the analysis of the sample before significant surface damage occurs. The samples were analysed using a He+ primary ion beam directed perpendicularly to the target surface at 3000 ± 5 eV energy with an analyser pass energy of 3000 eV. Low-energy sputtering was performed by 1 keV Ar+ bombardment at an angle of 59°. In the ESI,† we demonstrate that our LEIS results are representative of the undamaged ionic liquid surface, i.e. we operate under static conditions. In addition, the experimental reproducibility, the lack of surface charging, and the sample purity of all of our analysed LEIS samples are also demonstrated. Surface purity was routinely checked before recording LEIS spectra, and if any unexpected peaks were observed, Ar+ bombardment was carried out. More details are given in the ESI.†| Yi = NiPi+dσi/dΩA | (1) |
| Elements | Detection limit |
|---|---|
| Li to O | ≥1% |
| F To Cl | 1–0.05% |
How one defines which atoms are in contact with the vacuum is important; the probe used (and its properties) is vital to this definition. For example, the ionic liquid–vacuum outer atomic surface will be different if a relatively large probe such as Br2 is used compared to when a relatively small He+ ion probe is used. For [C2C1Im][NO3], hyperthermal O(3P) atoms were found to penetrate the ionic liquid–vacuum surface about 0.2 nm deeper than for hyperthermal Ar, most likely as the O atom has a smaller van der Waals radius than Ar by ∼20%.69 For LEIS, the He+ ion probe has a radius of ∼0.093 nm,91 although this estimate is based upon general considerations of ionic radii. Atomic and small molecular probes have also been used.46–51,70 Two groups have used intrinsic analysis MD simulations to investigate the composition of the ionic liquid–vacuum outer atomic surface; a probe with a radius of 0.2 nm was used, chosen to be close to the characteristic size of the atoms constituting the system.71–75
3. Results
We judge there are three potential scenarios to explain why an element is not at the ionic liquid–vacuum outer atomic surface. Firstly, the amount of this element in the bulk ionic liquid (i.e. the stoichiometric amount of that element in the ionic liquid) is relatively small. Secondly, the ions nearest to the vacuum are oriented such that the atom in question is covered by other atoms in the same ion. Thirdly, the ions nearest to the vacuum are covered by atoms in a different ion.3.1. Alkyl chains at the ionic liquid–vacuum outer atomic surface
All 23 ionic liquids studied here contain cationic carbon atoms; every [CnC1Im][A] ionic liquid contains five carbons bonded to a nitrogen atom (Chetero) and at least one carbon atom bonded only to carbon and hydrogen (Calkyl). In addition, there are also carbon atoms in seven of the anions (Canion) studied here ([Tf2N]−, [CF3SO3]−, [CnOSO3]−, [C1CO2]−, [SCN]−, [N(CN)2]−, [B(CN)4]−); 18 of the ionic liquids studied here contain at least one Canion. A Gaussian-shaped peak for carbon was observed for nine of the 23 ionic liquids. As the carbon peaks for the other 14 ionic liquids have the same peak energies (within experimental error) we conclude that carbon is at the outer atomic surface for all 23 ionic liquids. However, Ycarbon is small for all 23 ionic liquids, even for [P6,6,6,14]Cl, which contains almost exclusively Calkyl atoms and hydrogen atoms. For both [CnC1Im][Tf2N] and [C4C1Im][CnOSO3] Ycarbon does not increase as the alkyl chain length, n, is increased; Ycarbon for [C12C1Im][Tf2N] is the same (within experimental error) as Ycarbon for [C2C1Im][Tf2N].Reactive scattering experiments detect significantly more hydrogen atoms at the ionic liquid–vacuum outer atomic surface for [C12C1Im][Tf2N] than for [C2C1Im][Tf2N].46–48 The clear conclusion is that the [C12C1Im]+ cations are oriented such that on average Calkyl atoms are closer to the vacuum than Chetero for [C12C1Im][Tf2N]. For [C12C1Im][Tf2N], we can rule out Calkyl atoms being covered by atoms from the [Tf2N]− anion or atoms from the imidazolium ring. For [C4C1Im][PF6], using intrinsic analysis MD simulations it was shown that cationic carbon made up approximately 85% of the ionic liquid–vacuum outer atomic surface (excluding hydrogen atoms); the three imidazolium ring carbon atoms comprised ∼10%, and the alkyl carbons ∼75%.72 Our conclusion is that Ycarbon is relatively small in our LEIS experiments because Calkyl atoms are present just beneath the ionic liquid–vacuum outer atomic surface, with hydrogen atoms present at the ionic liquid–vacuum outer atomic surface (although dσcarbon/dΩ will also contribute to the relatively small Ycarbon). Overall, if the CH2 groups are considered as individual entities, they are certainly present in significant amounts for many ionic liquids at the ionic liquid–vacuum outer atomic surface.
3.2. Cation charged groups at the ionic liquid–vacuum outer atomic surface
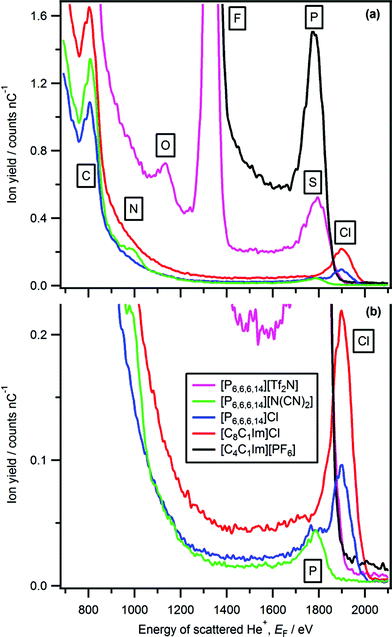
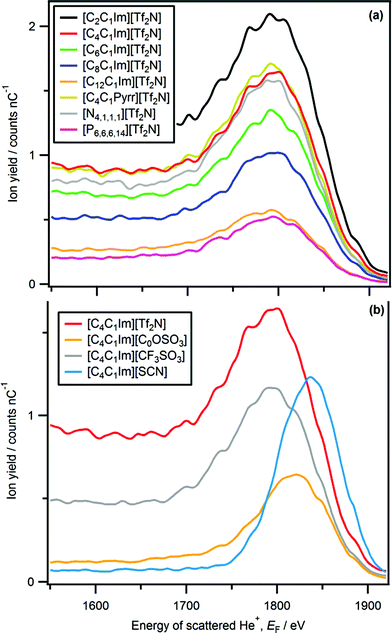
The only imidazolium-based ionic liquid for which a peak due to cationic nitrogen is observed was [C2C1Im][C1CO2] (Fig. 1 and 2). The nitrogen peak observed for [C2C1Im][C1CO2] has a very small Ynitrogen and cannot easily be seen in Fig. 1. However, in Fig. 2 a zoomed-in LEIS spectrum is shown, clearly showing the nitrogen peak for [C2C1Im][C1CO2]. No nitrogen peak was observed for two ammonium-based ionic liquids, [C4C1Pyrr][Tf2N] and [N4,1,1,1][Tf2N] (Fig. 3b). A very small phosphorus peak was observed for both [P6,6,6,14][N(CN)2] and [P6,6,6,14]Cl (Fig. 4b). It should be noted that it is difficult to determine if a phosphorus peak is present for [P6,6,6,14][Tf2N] as the sulfur peak of [Tf2N]− occurs in the same energy region, ∼1795 eV, as phosphorus.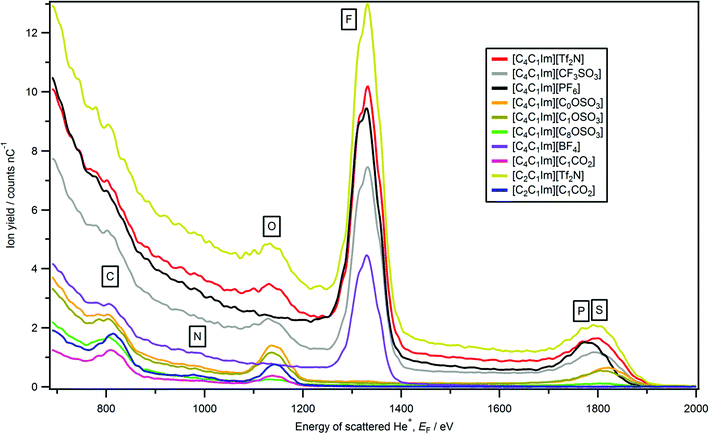 | ||
| Fig. 1 LEIS spectra (4He+, E0 = 3 keV) for eight [C4C1Im][A] and two [C2C1Im][A] ionic liquids. Ionic liquids which contain cyano groups (e.g. [C4C1Im][N(CN)2]) are not included in this plot (see Fig. 5 for LEIS spectra of cyano-containing ionic liquids). | ||
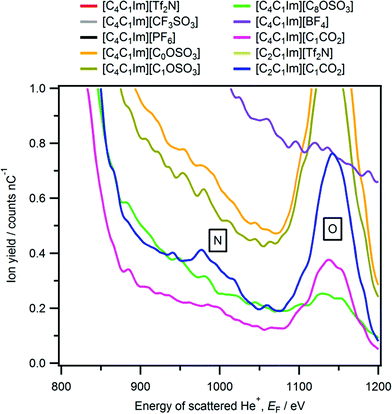 | ||
| Fig. 2 LEIS spectra (4He+, E0 = 3 keV) for eight [C4C1Im][A] and two [C2C1Im][A] ionic liquids. The energy range given is 800 eV to 1200 eV, with the y-scale is cropped to zoom in on the cationic nitrogen peak observed for [C2C1Im][C1CO2]. Ionic liquids which contain cyano groups (e.g. [C4C1Im][N(CN)2]) are not included in this plot (see Fig. 5 for LEIS spectra of cyano-containing ionic liquids). | ||
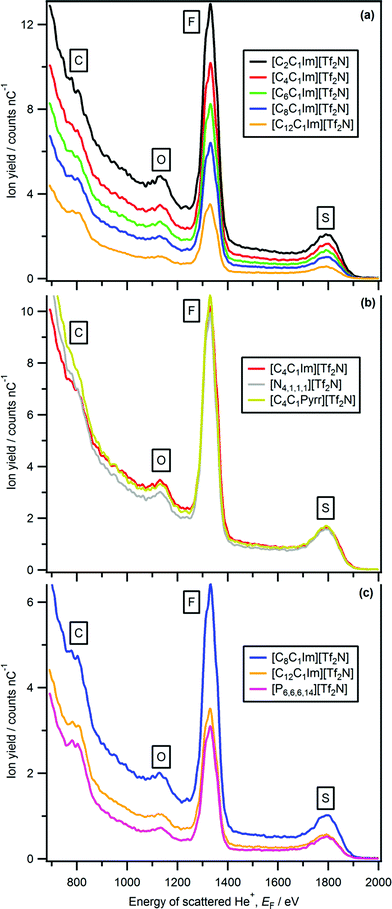 | ||
| Fig. 3 LEIS spectra (4He+, E0 = 3 keV) for: (a) [CnC1Im][Tf2N], (b) [C4C1Im][Tf2N], [C4C1Pyrr][Tf2N] and [N4,1,1,1][Tf2N], (c) [C8C1Im][Tf2N], [C12C1Im][Tf2N] and [P6,6,6,14][Tf2N]. | ||
For [C2C1Im][C1CO2] we conclude that cationic nitrogen is at the ionic liquid–vacuum outer atomic surface, as the nitrogen peak appears at the characteristic energy of a nitrogen surface peak (Table S3†). For the ionic liquids for which a nitrogen peak was not observed, the amount of imidazolium nitrogen atoms at the ionic liquid–vacuum outer atomic surface is <1–2% (Table 1). The stoichiometric amount of cationic nitrogen (for the ionic liquids shown in Fig. 1 and 3, ignoring the small hydrogen atoms) ranges from 4% for [C4C1Pyrr][Tf2N] and [N4,1,1,1][Tf2N] to 17% for [C2C1Im][C1CO2] (see Table S5† for more details). Clearly, the stoichiometric amount of nitrogen is far greater than the amount of nitrogen observed at the ionic liquid–vacuum outer atomic surface using LEIS. Using MD simulations, Hantal et al. for [C4C1Im][PF6] found that both nitrogen atoms were ∼1% each at the ionic liquid–vacuum outer atomic surface (excluding hydrogen atoms),72 agreeing with the conclusion here for [C4C1Im][PF6] that the imidazolium nitrogen atom percentage at the ionic liquid–vacuum outer atomic surface is <1–2%.
Cationic nitrogen was observed for the ionic liquid with the largest stoichiometric amount of cationic nitrogen, [C2C1Im][C1CO2] (at 17%). Two extra CH2 groups going from [C2C1Im][C1CO2] to [C4C1Im][C1CO2] (amount of cationic nitrogen = 14%) are sufficient to conceal the imidazolium nitrogen atoms. Therefore, a short alkyl chain is required for imidazolium nitrogen atoms to be observed at the ionic liquid–vacuum outer atomic surface, as the alkyl chains clearly cover the imidazolium nitrogen atoms. Changing the anion from [C2C1Im][C1CO2] to [C2C1Im][Tf2N] is sufficient to conceal the imidazolium nitrogen atoms. When considering anion size, the best method of assessment is the molecular volume, Vmol, which represents the volume occupied by one ionic liquid ion pair in the liquid phase. The [C1CO2]− anion is much smaller than the [Tf2N]− anion ([C2C1Im][C1CO2] Vmol = 0.257 nm3 and [C2C1Im][Tf2N] Vmol = 0.427 nm3, see ESI† for details of Vmol). The physical explanation for the importance of anion size to the amount of nitrogen at the ionic liquid–vacuum outer atomic surface is simple: when the anion is larger it occupies more of the ionic liquid–vacuum outer atomic surface, leaving less room for cations, leading to fewer cations being present at the ionic liquid–vacuum outer atomic surface.
Phosphorus peaks with small Yphosphorus are observed for both [P6,6,6,14][N(CN)2] and [P6,6,6,14]Cl. [P6,6,6,14][N(CN)2] and [P6,6,6,14]Cl contain 3% phosphorus atoms (excluding hydrogen atoms) but for ionic liquids with similar amounts of cationic heteroatoms at 4%, [C4C1Pyrr][Tf2N] and [N4,1,1,1][Tf2N], no nitrogen peak was observed. Therefore, we conclude that the stoichiometric amounts are not a key factor in the detection of phosphorus. An important factor is that the detection limit (most likely due to a combination of dσi/dΩ, Pi+ and a small scattered ion background) in LEIS for phosphorus is smaller than for nitrogen (Table 1). Another important factor is that phosphorus–carbon bonds (∼1.8 Å) are longer than nitrogen–carbon bonds (∼1.5 Å);92 therefore, phosphorus atoms are likely to be less covered by the four alkyl carbon atoms than the nitrogen atoms in the ammonium ionic liquids. At this stage, we cannot make further comment on the positions of the alkyl chains for these ionic liquids.
Lastly, as explained in Section 3.1, it is impossible to determine if a carbon peak is due to scattering from Chetero or Calkyl atoms, or indeed from Canion atoms. Therefore, no comments can be made on the amount of Chetero atoms present at the ionic liquid–vacuum outer atomic surface.
3.3. Cations located nearest to the vacuum have multiple orientations
Our finding that the amount of nitrogen at the ionic liquid–vacuum outer atomic surface is far below that expected from stoichiometry can be explained by considering the orientation of the [CnC1Im]+ cations nearest to the vacuum (although it must be noted we cannot directly determine the [CnC1Im]+ ring plane orientation from our LEIS experiments). Using SFG spectroscopy, Baldelli and co-workers have concluded that the [CnC1Im]+ ring plane is oriented parallel to the ionic liquid surface plane.1 For this [CnC1Im]+ ring orientation, we would expect both of the nitrogen atoms to be at the ionic liquid–vacuum outer atomic surface, and therefore would be detected using LEIS (see Fig. 6 in ref. 71 for a visualisation), giving an amount of nitrogen at the outer atomic surface similar to the stoichiometric amount. Based upon the upper limit for cationic nitrogen atoms at the ionic liquid–vacuum outer atomic surface, we find the [CnC1Im]+ ring orientation determined using SFG spectroscopy incompatible with our LEIS results. The three most common [C4C1Im]+ orientations proposed by Hantal et al. using MD simulations for [C4C1Im][PF6] were: alkyl chain towards the vacuum, [C4C1Im]+ ring plane parallel to surface plane = 16%; alkyl chain towards the vacuum and [C4C1Im]+ ring plane perpendicular to surface plane = 70%; alkyl chain towards the bulk and imidazolium ring plane perpendicular to surface plane = 7%.71 The first [CnC1Im]+ orientation, the same as proposed by Baldelli and co-workers, would likely lead to both nitrogen atoms of the ring being at the ionic liquid–vacuum outer atomic surface (see Fig. 6 in ref. 71 for a visualisation); the second and third [CnC1Im]+ orientations would mean that neither nitrogen atoms are present at the ionic liquid–vacuum outer atomic surface due to them being covered by other ring atoms. Our results are in agreement with the MD simulations, which suggests that the most probable orientation is with the [CnC1Im]+ ring plane is oriented perpendicular to the ionic liquid–vacuum surface plane; however, as we do detect nitrogen for [C2C1Im][C1CO2], there must be (for [C2C1Im][C1CO2] at least) multiple cation orientations.3.4. Anions at the ionic liquid–vacuum outer atomic surface
Peaks due to the anion are observed for all 23 ionic liquids studied here (Fig. 1, 3–5 and Table 2). All observed peaks are surface peaks, apart from for sulfur in [cation][Tf2N], [C4C1Im][CF3SO3] and [C4C1Im][CnOSO3], which contains both a surface peak and a sub-surface peak (Fig. 6). Therefore, it can be concluded that all elements observed, as listed in Table 2, are at the ionic liquid–vacuum outer atomic surface. Peaks not observed are: N for [cation][Tf2N], B for [C4C1Im][BF4] and [C2C1Im][B(CN)4]; we conclude that these elements are not at the ionic liquid–vacuum outer atomic surface in significant concentration. When considering the elements present at the outer atomic surface for each ionic liquid studied here, clearly all 23 ionic liquids have at least one strong hydrogen bond acceptor adsorption site.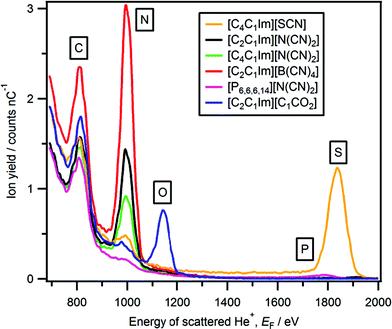 | ||
| Fig. 5 LEIS spectra (4He+, E0 = 3 keV) for which a nitrogen peak was observed ([C4C1Im][SCN], [C2C1Im][N(CN)2], [C4C1Im][N(CN)2], [C2C1Im][B(CN)4], [P6,6,6,14][N(CN)2] and [C2C1Im][C1CO2]). | ||
| Ionic liquid | B | C | N | O | F | P | S | Cl |
|---|---|---|---|---|---|---|---|---|
| [CnC1Im][C1CO2] | — | ? | — | Y | — | — | — | — |
| [cation]Cl | — | — | — | — | — | — | — | Y |
| [C4C1Im][SCN] | — | ? | Y | — | — | — | Y | — |
| [CnC1Im][N(CN)2] | — | ? | Y | — | — | — | — | — |
| [C2C1Im][B(CN)4] | N | ? | Y | — | — | — | — | — |
| [cation][Tf2N] | — | ? | N | Y | Y | — | Y (+Ssub-surface) | — |
| [C4C1Im][CF3SO3] | — | ? | — | Y | Y | — | Y (+Ssub-surface) | — |
| [C4C1Im][CnOSO3] | — | ? | — | Y | — | — | Y (+Ssub-surface) | — |
| [C4C1Im][BF4] | N | — | — | — | Y | — | — | — |
| [C4C1Im][PF6] | — | — | — | — | Y | Y | — | — |
 | ||
| Fig. 6 LEIS spectra (4He+, E0 = 3 keV) for sulfur-containing ionic liquids (region shown 1550 eV to 1920 eV): (a) [cation][Tf2N], (b) [C4C1Im][A]. | ||
Sulfur is at the ionic liquid–vacuum outer atomic surface for [C4C1Im][SCN], as a Gaussian-shaped sulfur peak was observed (Fig. 6); 1835 eV is used as a sulfur surface peak energy reference, labelled as Ssurface. The sulfur region for [cation][Tf2N], [C4C1Im][CF3SO3] and [C4C1Im][CnOSO3] (n = 0, 1, 8) gave a non-Gaussian shape (Fig. 6), with a considerable component at lower energy than the sulfur peak for [C4C1Im][SCN] (Fig. 6b). The shape of the sulfur region for [cation][Tf2N], [C4C1Im][CF3SO3] and [C4C1Im][CnOSO3] is very similar to the silicon region observed for SiO2 oxide, in which 90% of the silicon is covered in oxygen.93 Therefore, the lower energy component of the sulfur region is due to scattering from sulfur atoms located just below the outer atomic surface, which we label as Ssub-surface. This lower energy component is produced by neutralisation of He+ to form He0 on collision with Ssub-surface (and loss of a small amount of energy), followed by ionisation to re-form He+. We use Ssurface (1835 eV) as a constraint when fitting the sulfur region for [cation][Tf2N], and allow the peak energy of Ssub-surface to vary, as well as Ysulfur for both Ssub-surface and Ssurface. Peaks were obtained for both Ssub-surface and Ssurface for [cation][Tf2N], indicating that sulfur atoms are at the ionic liquid–vacuum outer atomic surface.
For [C4C1Im][PF6] phosphorus is at the ionic liquid–vacuum outer atomic surface, and therefore not completely covered by the six surrounding fluorine atoms, as a Gaussian-shaped phosphorus peak was observed at 1784 eV (Fig. 1). Hantal et al. found for [C4C1Im][PF6] that ∼13% of the ionic liquid–vacuum outer atomic surface was composed of fluorine atoms, and ∼1% of phosphorus atoms.72 However, Hantal et al. used a probe (radius 0.2 nm) approximately double in size to our He+ ion probe (radius 0.093 nm);91 hence our findings are not contradictory.
There are several literature studies that conclude that the anion is not at the ionic liquid–vacuum outer atomic surface for ionic liquids containing long alkyl chains, as the anions are covered by alkyl chains.35,36,53–57 We have studied the same/similar ionic liquids, and found part/all of the anions at the ionic liquid–vacuum outer atomic surfaces. As the interpretation of our LEIS results is unambiguous, in the following section we attempt to reconcile these apparent contradictions. Using MD simulations, Pensado et al. concluded for [C6C1Im][Tf2N] and [C8C1Im][BF4] that the long alkyl chain sticks out into vacuum, but their density is significantly lower than close alkyl packing.65,66 Hence, it was qualitatively judged that small amounts of anions are at the ionic liquid–vacuum outer atomic surface as the alkyl groups do not cover them completely, in agreement with our results. Iwahashi et al. used MAES and calculations to conclude that for [C10C1Im][Tf2N], the [Tf2N]− anion is at the ionic liquid–vacuum outer atomic surface.35 A similar conclusion has been reached using atom scattering/reactivity and MD simulations for [C12C1Im][Tf2N].46–48 This observation is in agreement with our results that the [Tf2N]− anion is at the ionic liquid–vacuum outer atomic surface for [C12C1Im][Tf2N]. Hammer et al. used NICISS to conclude, for [C8C1Im][Tf2N], that no fluorine is at the ionic liquid–vacuum outer atomic surface as the octyl chain covers the anion.53 This conclusion was based upon carbon and fluorine depth profiles that have maxima (relative to the ionic liquid–vacuum outer atomic surface) at 3 Å (due to the octyl chain) and 7 Å (due to the [Tf2N]− anion) respectively.53 We expect the alkyl chains to have a density that is lower than close packing, as demonstrated by Pensado et al.,65,66 and consequently, anions are at the ionic liquid–vacuum outer atomic surface as the alkyl chains are unable to obscure all anions. Therefore, our results do not contradict the results of Hammer et al., but we do contradict their interpretation of their results. It should be noted that Kimura and co-workers concluded from RBS for [C4C1Im][PF6] and [CnC1Im][Tf2N] that the alkyl chains protrude from the bulk liquid to the vacuum, with imidazolium nitrogen and anionic atoms just beneath the alkyl chains.52,58 Again, we conclude for [C4C1Im][PF6] and [CnC1Im][Tf2N] that anions are at the ionic liquid–vacuum outer atomic surface as the alkyl chains are unable to obscure all anions. Reichelt et al. used NICISS for [C6C1Im]Cl to conclude that there are hexyl chains located nearer the vacuum than Cl− anions, and that the ionic liquid–vacuum outer atomic surface is composed of cationic atoms.54 Ulbrich et al. used MAES and calculations to conclude that Cl− is at the solid–vacuum outer atomic surface for CsCl but not at the ionic liquid–vacuum outer atomic surface for [C8C1Im]Cl.36 Our results show that Cl− is definitely present at the ionic liquid–vacuum outer atomic surface for [C8C1Im]Cl (Fig. 4). Therefore, for [C8C1Im]Cl we conclude that octyl chains are not present in sufficient density to completely cover the Cl− anion, as we have also established for [C12C1Im][Tf2N].
3.5. Anions located nearest to the vacuum have multiple orientations
To date, it has been common to report only the most probable ion orientations.1,2,28–39,46–48,52–57 From our LEIS results we cannot directly compare Yi for different elements as dσi/dΩ and Pi+ are unknown at this stage. Therefore, we cannot determine the relative amounts of different anionic elements at the outer atomic surface, and therefore cannot draw conclusions on the relative amounts of different anion orientations. However, we can deduce if there are multiple anion orientations for those anions nearest to the vacuum for [Tf2N]−, [CF3SO3]−, [CnOSO3]−, [SCN]− and [C1CO2]−.Most reports on anion orientation are for [cation][Tf2N].29,35,52,59,65,70,73,77 Whilst dσi/dΩ and Pi+ are unknown at this stage, we are confident that the very intense fluorine peak for all eight [cation][Tf2N] ionic liquids investigated here indicates that for the [Tf2N]− anions nearest to the vacuum, the most probable orientation is with CF3 groups towards the vacuum. However, it is clear that the SO2 groups are also at the ionic liquid–vacuum outer atomic surface, confirming that there are multiple [Tf2N]− orientations for those anions nearest to the vacuum. Our results for the most probable [Tf2N]− orientation for the anions nearest to the vacuum are in agreement with conclusions in the literature.29,35,52,59,65,70,73,77 Kimura and co-workers used RBS to suggest the possibility of more than one anion orientation for [C6C1Im][Tf2N], but not for [C2C1Im][Tf2N].52 Our evidence confirms the presence of multiple anion orientations for [Tf2N]− for all eight cations studied here, including [C2C1Im][Tf2N].
As for [cation][Tf2N], by far the most intense peak in the LEIS spectrum for [C4C1Im][CF3SO3] is from fluorine, indicating that the most probable orientation for the [CF3SO3]− anions is with CF3 group towards the vacuum, in agreement with the anion orientation proposed by Iwahashi et al. using SFG spectroscopy.34 However, our LEIS results clearly show that the SO3 group is also at the ionic liquid–vacuum outer atomic surface for [C4C1Im][CF3SO3], confirming that there are multiple [CF3SO3]− orientations for those anions nearest to the vacuum.
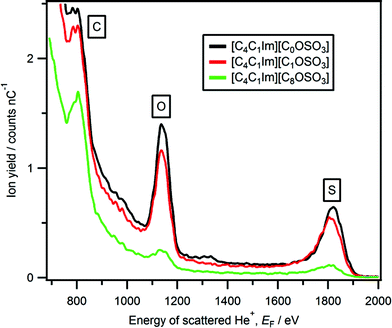
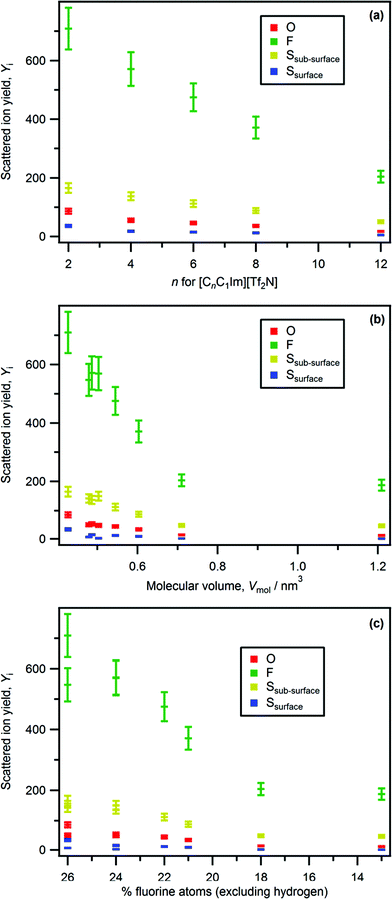
From our LEIS studies, for [C4C1Im][CnOSO3] (n = 0, 1, and 8) as n is increased both Yoxygen and Ysulfur(surface) decrease more than expected based upon stoichiometry (Fig. 7 and Table S4†). The decrease in the stoichiometric amount of oxygen in [C4C1Im][CnOSO3] for n = 0 to n = 8 is 27–17% (see Table S5† for more details on amounts of each element); the associated decrease in Yoxygen for n = 0 to n = 8 is 78 to 11. The decrease in the stoichiometric amount of sulfur in [C4C1Im][CnOSO3] for n = 0 to n = 8 is 7–4%; the associated decrease in Ysulfur(surface) for n = 0 to n = 8 is 19 to 2. These large decreases in Yoxygen and Ysulfur(surface) are due to the orientation of the anions nearest to the vacuum. Clearly, the most probable anion orientation is with the anionic alkyl chain closer to the vacuum and the sulfate group closer to the bulk liquid, in agreement with experimental and MD simulations studies on [C2C1Im][C8OSO3].29,30,67 However, the fact that peaks due to oxygen and sulfur are detected for [C4C1Im][CnOSO3], even when n = 8, demonstrates that there are multiple anion orientations for the [CnOSO3]− anions nearest to the vacuum.
For [C4C1Im][SCN] both anionic nitrogen and sulfur atoms are at the ionic liquid–vacuum outer atomic surface; hence, we conclude that there are multiple anion orientations. One can envisage two extremes of anion orientation for the [SCN]− anions nearest to the vacuum: the [SCN]− anion is always oriented with the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds approximately perpendicular to ionic liquid–vacuum surface plane with S towards the vacuum and N towards the bulk, and vice versa. Further work is required to determine which of these two scenarios is the most probable anion orientation at the ionic liquid–vacuum surface for [C4C1Im][SCN].
N bonds approximately perpendicular to ionic liquid–vacuum surface plane with S towards the vacuum and N towards the bulk, and vice versa. Further work is required to determine which of these two scenarios is the most probable anion orientation at the ionic liquid–vacuum surface for [C4C1Im][SCN].
Based upon experimental studies for other ionic liquids,1,2,28–39,46–48,52–58 one can assume that the most probable anion orientation for [CnC1Im][C1CO2] is with the CH3 group of [C1CO2]− towards the vacuum and the CO2 groups towards the bulk; this conclusion has also been reached using MD simulations for [C4C1Im][C1CO2].78 However, for [C4C1Im][C1CO2] anionic oxygen atoms are at the ionic liquid–vacuum outer atomic surface. Therefore, we conclude that for [C1CO2]− anions nearest to the vacuum there are multiple anion orientations. We expect that the most probable anion orientation is with the CH3 group of [C1CO2]− towards the vacuum and the CO2 group towards the bulk liquid, but clearly the oxygen atoms provide strong hydrogen bond acceptor adsorption sites.
Overall, for [Tf2N]−, [CF3SO3]−, [CnOSO3]−, [SCN]− and [C1CO2]− we found multiple anion orientations for those anions nearest the vacuum. This finding has not been reported to date for ionic liquid anions, apart from one report from Kimura and co-workers for [C6C1Im][Tf2N], although it must be noted that their evidence is not strong.52
3.6. Tuning the amount of anion at the ionic liquid–vacuum outer atomic surface by varying the cation
Using anionic Yi we can determine the effect of the cation on the relative amounts of anionic elements, and therefore amounts of anion, at the ionic liquid–vacuum outer atomic surface. Two cationic variables can be considered: the alkyl chain length for the same cation core, and the cation core itself. We compare Yi to the expected stoichiometric amount of each atom in the bulk ionic liquid to judge the effect of the cation on the anion (see Table S5† for more details).For [CnC1Im][Tf2N], as n was increased Yi for all anion peaks (i.e. O, F, Ssub-surface, Ssurface) decreased significantly greater than expected from stoichiometry (Fig. 3 and 8a). Therefore, there is far less anion at the ionic liquid–vacuum outer atomic surface than expected from stoichiometry as n increased. From [C2C1Im][Tf2N] to [C12C1Im][Tf2N], Yfluorine decreased from 709 to 204; the stoichiometric decrease in fluorine for these ionic liquids is from 26% to 18%. In addition, from [C2C1Im][Tf2N] to [C12C1Im][Tf2N], Yoxygen decreased from 86 to 17 (stoichiometric decrease from 26% to 18%) and Ysulfur(surface) decreased from 36 to 5 (stoichiometric decrease from 9% to 6%). We conclude that as n increased, the anions were increasingly covered by the alkyl chains, but certainly not completely covered, in agreement with previous reports based upon atom scattering/reactivity experiments.46–48
Y nitrogen is significantly greater for [C2C1Im][N(CN)2] than [C4C1Im][N(CN)2]. Yoxygen is approximately double for [C2C1Im][C1CO2] compared to [C4C1Im][C1CO2]. For both of these families of ionic liquids, Yanion decreased as n is increased, indicating that the anions were partially covered by the alkyl chains, but certainly not completely covered.
Y nitrogen for [C2C1Im][N(CN)2] and [P6,6,6,14][N(CN)2] are very different indeed, 72 and 4 respectively. This observation indicates that there is approximately 18 times as much nitrogen at the ionic liquid–vacuum outer atomic surface for [C2C1Im][N(CN)2] than for [P6,6,6,14][N(CN)2]. For [C2C1Im][N(CN)2] and [P6,6,6,14][N(CN)2], the percentage of anionic nitrogen is 38% and 8% respectively. Clearly, the decrease in anion quantity from [C2C1Im][N(CN)2] to [P6,6,6,14][N(CN)2] is greater than expected simply by stoichiometry alone, indicating that the anions for [P6,6,6,14][N(CN)2] were substantially covered by the alkyl chains, but not completely covered.
Y chlorine for [C8C1Im]Cl is approximately double Ychlorine for [P6,6,6,14]Cl; the values are 21 and 9 respectively. This observation indicates that there is approximately twice as much chlorine at the ionic liquid–vacuum outer atomic surface for [C8C1Im]Cl than for [P6,6,6,14]Cl. For [C8C1Im]Cl and [P6,6,6,14]Cl, the percentage of anionic nitrogen is 7% and 3% respectively. This decrease in Cl− anion quantity is approximately that expected based upon stoichiometry.
For [C4C1Im][Tf2N], [C4C1Pyrr][Tf2N] and [N4,1,1,1][Tf2N] Yoxygen, Yfluorine and Ysulfur are very similar (Fig. 3b, 8b, c and Table S4†). This observation indicates that there is approximately the same amount of [Tf2N]− anion at the ionic liquid–vacuum outer atomic surface for [C4C1Im][Tf2N], [C4C1Pyrr][Tf2N] and [N4,1,1,1][Tf2N]. All three of these ionic liquids have very similar Vmol and also have the same largest alkyl chain, a butyl. In addition, all three of these ionic liquids have ∼25% fluorine atoms.
For [C12C1Im][Tf2N] and [P6,6,6,14][Tf2N] Yi for oxygen, fluorine and sulfur are very similar (Fig. 3c, 8b, c and Table S4†). This observation indicates that there is approximately the same amount of [Tf2N]− anion at the ionic liquid–vacuum outer atomic surface for [C12C1Im][Tf2N] and [P6,6,6,14][Tf2N]. These ionic liquids have very different Vmol and different amounts of alkyl carbon. In addition, they have 18% and 13% fluorine atoms respectively.
The amount of anion at the ionic liquid–vacuum outer atomic surface is clearly affected by the cation. In broad terms, ionic liquids with longer alkyl chains have less anion at the ionic liquid–vacuum outer atomic surface. This trend can also be explained in terms of cation size: ionic liquids with larger cations have less anion at the ionic liquid–vacuum outer atomic surface. For most ionic liquids considered, the decrease in anion quantity with increasing alkyl chain quantity is far greater than expected based upon just stoichiometry. There are two possible explanations for this finding: firstly, the increased probability of alkyl carbon being at the ionic liquid–vacuum outer atomic surface as n increases, and secondly, changes in the orientation of the anions nearest to the vacuum as n increases. Based upon a range of experiments (e.g. ARXPS30–33 and atom scattering/reactivity46–48) we conclude that the first explanation is far more credible. It must be noted that the results for [P6,6,6,14][A] demonstrate that the amount of anion cannot be explained with reference to just one variable; neither the amount of alkyl carbon in the bulk ionic liquid nor the anion size correlate with the amount of anion at the ionic liquid–vacuum outer atomic surface. Due to a lack of comparable data from other methods, no speculation will be made on the surface structure of [P6,6,6,14][A] ionic liquids.
4. Overall discussion
Based upon our LEIS results and literature findings, it is clear that at least part of both the cation and the anion are present in the ionic liquid–vacuum outer atomic surface of all 23 ionic liquids investigated. Anionic elements are at the ionic liquid–vacuum outer atomic surface, as the alkyl chains do not form a high density overlayer and therefore do not completely cover the anions, even for ionic liquids which are almost exclusively composed of alkyl chains (e.g. [P6,6,6,14]Cl). Consequently, it must be concluded that thinking of the ionic liquid–vacuum surface as consisting of an alkyl overlayer and an ionic underlayer is an oversimplification. All 23 ionic liquids studied here have at least one element at the outer atomic surface that provides a strong hydrogen bond acceptor adsorption site. Therefore, there are relatively energetically favourable adsorption sites for many important adsorbates, e.g. water, and clearly all adsorption sites at the ionic liquid–vacuum surface are not equivalent. The realisation that the anions are always observed at the ionic liquid–vacuum outer atomic surface has allowed us to re-evaluate and at times reinterpret the published data of other groups. It is important to note that we have studied the ionic liquids with the longest alkyl chains that are liquid at room temperature, and observed anions at the ionic liquid–vacuum outer atomic surface for all of them. Therefore, it is difficult to envisage a scenario where the anion is completely covered (i.e. <1% of elements) at the ionic liquid–vacuum outer atomic surface. However, we do not observe all elements within these ions, e.g. we do not observe a nitrogen peak for [cation][Tf2N]. We estimate that the amount of cationic nitrogen at the ionic liquid–vacuum outer atomic surface is <2% of the elements at the ionic liquid–vacuum outer atomic surface for all of the imidazolium- and ammonium-containing ionic liquids studied here, apart from [C2C1Im][C1CO2]. For all [cation][Tf2N] we also estimate that the amount of anionic nitrogen at the ionic liquid–vacuum outer atomic surface is <2%. All other elements (apart from boron) are observed. Kauling et al. used LEIS to investigate the distribution of gold nanoparticles in [C4C1Im][PF6] and four functionalised ionic liquids.81 For the neat ionic liquids, they concluded that the ionic liquid–vacuum outer atomic surface is composed of all atoms of both cations and anions for all ionic liquids studied. However, we cannot observe a characteristic nitrogen peak in any of the LEIS spectra of those five ionic liquids (Fig. 2 in ref. 81). Therefore, the conclusion that the ionic liquid–vacuum outer atomic surface is composed of all atoms of both cations and anions for those ionic liquids is, from our point of view, incorrect.To maximise the amount of charged cationic groups at the ionic liquid–vacuum outer atomic surface, the crucial factors are a short cationic alkyl chain and a small anion. To maximise the amount of anion at the ionic liquid–vacuum outer atomic surface, size matters: the cation must be as small as possible. This knowledge can be used to tune the amount of cation (or anion) at the ionic liquid–vacuum outer atomic surface. More subtle factors must be considered when the ion sizes are similar for two ionic liquids; at present we do not have sufficient data to identify these factors.
For both cations and anions nearest to the vacuum, multiple ion orientations clearly occur. To date, most studies of the ionic liquid–vacuum surface report the most probable ion orientations, which has led to the model outlined in our introduction that the alkyl chains are nearer to the vacuum and the charged groups are nearer the bulk than the alkyl chains is the most probable composition. However, other orientations occur. Due to the liquid nature of ionic liquids, we envisage that there are a wide range of anion orientations occurring, as ions diffuse and collide at the ionic liquid–vacuum surface. However, it is clearly of interest to try to understand which are the most probable anion orientations; further studies are evidently required to obtain a fuller picture of ion orientations. The conclusion that multiple ion orientations occur is important as the less probable orientations may be responsible for observed phenomena from kinetics studies,60–62 molecular scattering,51 CO2 capture,9 and nanoparticle preparation,94 for which the interpretation relies heavily upon studies of the ionic liquid–vacuum surface structure. In particular, the presence of relatively reactive groups, e.g. halide anions, the carboxylate group of [C1CO2]−, and the sulfate group of [C0OSO3]−, is expected to have an impact upon applications of the ionic liquid–gas surface, e.g. gas capture/storage/separation,3–8 nanoparticle and thin film preparation,10–14 supported ionic liquid phase (SILP) catalysis,15,16 and stationary phases for gas chromatography.17,18
Three examples are given where the presence of multiple ion orientations may help interpret the data. The thermally averaged kinetic parameters are very similar for water adsorption/desorption from [C8C1Im][BF4] and [C2C1Im][Tf2N].60–62 These findings are surprising, if one considers the most probable surface composition (where the ionic liquid–vacuum outer atomic surface for [C8C1Im][BF4] is dominated by alkyl chains and the ionic liquid–vacuum outer atomic surface for [C2C1Im][Tf2N] is dominated by relatively reactive anions). However, for both [C8C1Im][BF4] and [C2C1Im][Tf2N] anionic moieties that interact relatively strongly with water, e.g. BF and SO2 respectively, will be at the ionic liquid–vacuum outer atomic surface due to the alkyl chains not completely covering the [BF4]− (or indeed the [Tf2N]−) and the [Tf2N]− having multiple anion orientations. Ziemkiewich et al. investigated NO molecular beam scattering from three ionic liquids, [C4C1Im][A] ([A]− = Cl−, [BF4]−, and [Tf2N]−).51 A systematic increase in final rotational energy was observed with increasing anion size. To explain these molecular scattering findings, one needs to consider the changes that will occur to the ionic liquid–vacuum surface when the anion is changed from Cl− to [Tf2N]−. This observation could be due to differences in scattering from the different anions. An additional consideration is the multiple anion orientations that [Tf2N]−, unlike Cl− and [BF4]−, can adopt for those anions nearest the ionic liquid–vacuum outer atomic surface. When the anion is changed from Cl− to [Tf2N]−, the amount of cationic atoms at the outer atomic surface will decrease. Again, considerations of cation orientation need to be made. Martinez et al. have measured the ionic liquid–vacuum surface potential for 10 [CnC1Im][CnOSO3] ionic liquids (where n = 1 to 8).42 These results were interpreted using a model of an alkyl overlayer and an ionic underlayer. It should be noted that we have detected the OSO3 group at the ionic liquid–vacuum outer atomic surface for [C4C1Im][C8OSO3]. As explained previously, such a model is an oversimplification and a more complex model is required to explain these findings.
Given the complexity of ionic liquid–vacuum surfaces, MD simulations have a vital role to play in gaining a clearer understanding. To date quantitative validation of MD simulations has focused mainly upon experimental and calculated values of surface tension.45,64–66 Our results can be used for quantitative validation of MD simulations of ionic liquid–vacuum surfaces. For example, from our results, one knows the relative amounts of fluorine present at the ionic liquid–vacuum outer atomic surface for [C2C1Im][Tf2N] and [C12C1Im][Tf2N]. Our Yi values can be directly compared to values obtained using MD simulations. A comparison has been made in this paper for LEIS and MD simulation data72 for [C4C1Im][PF6], but there is scope for molecular level validation of a large number of ionic liquids, if the number of atoms located at the ionic liquid–vacuum outer atomic surface from MD simulations is reported.
5. Conclusions
High-sensitivity LEIS has allowed us to study the elements at the ionic liquid–vacuum outer atomic surface. Our sample set comprises 23 carefully selected “target” ionic liquids to attempt to capture the common ionic liquid structural moieties. We have combined our results with literature data from different techniques and simulations in order to elucidate the surface structure of ionic liquids and the factors that affect its formation. This work provides a new, updated picture of the ionic liquid–vacuum surface with an unprecedented level of detail.The composition of the ionic liquid–vacuum outer atomic surface is more complex than suggested by many previous studies. We find that the probability of cationic heteroatoms being at the ionic liquid–vacuum outer atomic surface is very low. We have established that part/all of the anion is at the ionic liquid–vacuum outer atomic surface for all 23 ionic liquids studied; for ionic liquids with long alkyl chains, these chains dominate the ionic liquid–vacuum outer atomic surface, but are not sufficiently densely packed to completely cover the anions. The presence of strong hydrogen bond acceptor adsorption sites has important implications for understanding adsorption, especially when considering adsorbates which can act as hydrogen bond donors, e.g. water and ethanol. We demonstrate that the amount of an ion present at the ionic liquid–vacuum outer atomic surface can be tuned by varying the size of the other ion; larger cations (or anions) occupy more of the ionic liquid–vacuum outer atomic surface, leaving less room for anions (or cations). By identifying elements present at the ionic liquid–vacuum outer atomic surface, conclusions can be drawn on the orientations of anions nearest the vacuum. For both cation and anions multiple orientations are observed, with some anion orientations having a higher probability than others. To date most experimental and simulation studies report, for the ions nearest to the vacuum, the most probable ion orientations, and do not report multiple ion orientations. Thus, the models developed are not representative of ionic liquid–vacuum surfaces. We propose that these findings are particularly important, as the less probable ion orientations may dominate the observed surface properties.
Acknowledgements
KRJL acknowledges Imperial College London for the award of a Junior Research Fellowship, and thanks Prof. Tom Welton for his help and excellent guidance. SF acknowledges support from the EPRSC, EP/H006060/1 for the purchase of the LEIS instrument. IJVG acknowledge support from the EPSRC, EP/J021199/1.References
- C. S. Santos and S. Baldelli, Chem. Soc. Rev., 2010, 39, 2136–2145 RSC.
- K. R. J. Lovelock, Phys. Chem. Chem. Phys., 2012, 14, 5071–5089 RSC.
- L. A. Blanchard, D. Hancu, E. J. Beckman and J. F. Brennecke, Nature, 1999, 399, 28–29 CrossRef PubMed.
- J. L. Anderson, J. K. Dixon and J. F. Brennecke, Acc. Chem. Res., 2007, 40, 1208–1216 CrossRef CAS PubMed.
- A. Heintz, J. Chem. Thermodyn., 2005, 37, 525–535 CrossRef CAS PubMed.
- X. Han and D. W. Armstrong, Acc. Chem. Res., 2007, 40, 1079–1086 CrossRef CAS PubMed.
- C. F. Poole, J. Chromatogr. A, 2004, 1037, 49–82 CrossRef CAS PubMed.
- C. F. Poole and S. K. Poole, J. Sep. Sci., 2011, 34, 888–900 CrossRef CAS PubMed.
- I. Niedermaier, M. Bahlmann, C. Papp, C. Kolbeck, W. Wei, S. K. Calderon, M. Grabau, P. S. Schulz, P. Wasserscheid, H. P. Steinruck and F. Maier, J. Am. Chem. Soc., 2014, 136, 436–441 CrossRef CAS PubMed.
- J. Dupont and J. D. Scholten, Chem. Soc. Rev., 2010, 39, 1780–1804 RSC.
- H. Wender, R. V. Goncalves, A. F. Feil, P. Migowski, F. S. Poletto, A. R. Pohlmann, J. Dupont and S. R. Teixeira, J. Phys. Chem. C, 2011, 115, 16362–16367 CAS.
- T. Torimoto, T. Tsuda, K. Okazaki and S. Kuwabata, Adv. Mater., 2010, 22, 1196–1221 CrossRef CAS PubMed.
- S. Kuwabata, T. Tsuda and T. Torimoto, J. Phys. Chem. Lett., 2010, 1, 3177–3188 CrossRef CAS.
- E. F. Borra, O. Seddiki, R. Angel, D. Eisenstein, P. Hickson, K. R. Seddon and S. P. Worden, Nature, 2007, 447, 979–981 CrossRef CAS PubMed.
- A. Riisager, R. Fehrmann, M. Haumann and P. Wasserscheid, Top. Catal., 2006, 40, 91–102 CrossRef CAS PubMed.
- A. Riisager, R. Fehrmann, M. Haumann and P. Wasserscheid, Eur. J. Inorg. Chem., 2006, 695–706 CrossRef CAS.
- A. Marciniak, Int. J. Mol. Sci., 2010, 11, 1973–1990 CrossRef CAS PubMed.
- A. Marciniak, Int. J. Mol. Sci., 2011, 12, 3553–3575 CrossRef CAS PubMed.
- C. Kolbeck, J. Lehmann, K. R. J. Lovelock, T. Cremer, N. Paape, P. Wasserscheid, A. P. Fröba, F. Maier and H. P. Steinrück, J. Phys. Chem. B, 2010, 114, 17025–17036 CrossRef CAS PubMed.
- M. Tariq, M. G. Freire, B. Saramago, J. A. P. Coutinho, J. N. Canongia Lopes and L. P. N. Rebelo, Chem. Soc. Rev., 2012, 41, 829–868 RSC.
- N. V. Plechkova and K. R. Seddon, Chem. Soc. Rev., 2008, 37, 123–150 RSC.
- R. Giernoth, Angew. Chem., Int. Ed., 2010, 49, 2834–2839 CrossRef CAS PubMed.
- B. Winter and M. Faubel, Chem. Rev., 2006, 106, 1176–1211 CrossRef CAS PubMed.
- P. B. Petersen and R. J. Saykally, in Annu. Rev. Phys. Chem., Annual Reviews, Palo Alto, 2006, vol. 57, pp. 333–364 Search PubMed.
- K. R. J. Lovelock and P. Licence, in Ionic Liquids UnCOILed: Critical Expert Overviews, ed. K. R. Seddon and N. V. Plechkova, Wiley, Oxford, 2012, pp. 251–282 Search PubMed.
- C. Waring, P. A. J. Bagot, M. L. Costen and K. G. McKendrick, J. Phys. Chem. Lett., 2011, 2, 12–18 CrossRef CAS.
- C. Andersson and C. Ridings, Chem. Rev., 2014 DOI:10.1021/cr400417f.
- V. Lockett, R. Sedev, C. Bassell and J. Ralston, Phys. Chem. Chem. Phys., 2008, 10, 1330–1335 RSC.
- V. Lockett, R. Sedev, S. Harmer, J. Ralston, M. Horne and T. Rodopoulos, Phys. Chem. Chem. Phys., 2010, 12, 13816–13827 RSC.
- K. R. J. Lovelock, C. Kolbeck, T. Cremer, N. Paape, P. S. Schulz, P. Wasserscheid, F. Maier and H. P. Steinrück, J. Phys. Chem. B, 2009, 113, 2854–2864 CrossRef CAS PubMed.
- C. Kolbeck, T. Cremer, K. R. J. Lovelock, N. Paape, P. S. Schulz, P. Wasserscheid, F. Maier and H. P. Steinrück, J. Phys. Chem. B, 2009, 113, 8682–8688 CrossRef CAS PubMed.
- F. Maier, T. Cremer, C. Kolbeck, K. R. J. Lovelock, N. Paape, P. S. Schulz, P. Wasserscheid and H. P. Steinrück, Phys. Chem. Chem. Phys., 2010, 12, 1905–1915 RSC.
- S. Men, B. B. Hurisso, K. R. J. Lovelock and P. Licence, Phys. Chem. Chem. Phys., 2012, 14, 5229–5238 RSC.
- T. Iwahashi, T. Miyamae, K. Kanai, K. Seki, D. Kim and Y. Ouchi, J. Phys. Chem. B, 2008, 112, 11936–11941 CrossRef CAS PubMed.
- T. Iwahashi, T. Nishi, H. Yamane, T. Miyamae, K. Kanai, K. Seki, D. Kim and Y. Ouchi, J. Phys. Chem. C, 2009, 113, 19237–19243 CAS.
- A. Ulbrich, M. Reinmoller, W. J. D. Beenken and S. Krischok, ChemPhysChem, 2012, 13, 1718–1724 CrossRef CAS PubMed.
- O. Höfft, S. Bahr, M. Himmerlich, S. Krischok, J. A. Schaefer and V. Kempter, Langmuir, 2006, 22, 7120–7123 CrossRef PubMed.
- S. Krischok, R. Öttking, W. J. D. Beenken, M. Himmerlich, P. Lorenz, O. Höfft, S. Bahr, V. Kempter and J. A. Schaefer, Z. Phys. Chem., 2006, 220, 1407–1416 CrossRef CAS.
- S. Krischok, M. Eremtchenko, M. Himmerlich, P. Lorenz, J. Uhlig, A. Neumann, R. Öttking, W. J. D. Beenken, O. Höfft, S. Bahr, V. Kempter and J. A. Schaefer, J. Phys. Chem. B, 2007, 111, 4801–4806 CrossRef CAS PubMed.
- C. Aliaga, G. A. Baker and S. Baldelli, J. Phys. Chem. B, 2008, 112, 1676–1684 CrossRef CAS PubMed.
- I. S. Martinez and S. Baldelli, J. Phys. Chem. C, 2010, 114, 11564–11575 CAS.
- I. S. Martinez, C. Santos and S. Baldelli, ChemPhysChem, 2012, 13, 1818–1824 CrossRef CAS PubMed.
- Y. Jeon, J. Sung, W. Bu, D. Vaknin, Y. Ouchi and D. Kim, J. Phys. Chem. C, 2008, 112, 19649–19654 CAS.
- E. Sloutskin, B. M. Ocko, L. Tamam, I. Kuzmenko, T. Gog and M. Deutsch, J. Am. Chem. Soc., 2005, 127, 7796–7804 CrossRef PubMed.
- E. Sloutskin, R. M. Lynden-Bell, S. Balasubramanian and M. Deutsch, J. Chem. Phys., 2006, 125, 174715 CrossRef CAS PubMed.
- C. Waring, P. A. J. Bagot, J. M. Slattery, M. L. Costen and K. G. McKendrick, J. Phys. Chem. A, 2010, 114, 4896–4904 CrossRef CAS PubMed.
- C. Waring, P. A. J. Bagot, J. M. Slattery, M. L. Costen and K. G. McKendrick, J. Phys. Chem. Lett., 2010, 1, 429–433 CrossRef CAS.
- B. H. Wu, J. M. Zhang, T. K. Minton, K. G. McKendrick, J. M. Slattery, S. Yockel and G. C. Schatz, J. Phys. Chem. C, 2010, 114, 4015–4027 CAS.
- J. R. Roscioli and D. J. Nesbitt, J. Phys. Chem. Lett., 2010, 1, 674–678 CrossRef CAS.
- J. R. Roscioli and D. J. Nesbitt, J. Phys. Chem. A, 2011, 115, 9764–9773 CrossRef CAS PubMed.
- M. P. Ziemkiewich, A. Zutz and D. J. Nesbitt, J. Phys. Chem. C, 2012, 116, 14284–14294 Search PubMed.
- K. Nakajima, A. Ohno, H. Hashimoto, M. Suzuki and K. Kimura, J. Chem. Phys., 2010, 133, 044702 CrossRef PubMed.
- T. Hammer, M. Reichelt and H. Morgner, Phys. Chem. Chem. Phys., 2010, 12, 11070–11080 RSC.
- M. Reichelt, T. Hammer and H. Morgner, Surf. Sci., 2011, 605, 1402–1411 CrossRef CAS PubMed.
- C. Ridings, V. Lockett and G. Andersson, Phys. Chem. Chem. Phys., 2011, 13, 17177–17184 RSC.
- C. Ridings, V. Lockett and G. Andersson, Phys. Chem. Chem. Phys., 2011, 13, 21301–21307 RSC.
- C. Ridings, V. Lockett and G. Andersson, Colloids Surf., A, 2012, 413, 149–153 CrossRef CAS PubMed.
- A. Ohno, H. Hashimoto, K. Nakajima, M. Suzuki and K. Kimura, J. Chem. Phys., 2009, 130, 204705 CrossRef PubMed.
- S. Caporali, U. Bardi and A. Lavacchi, J. Electron Spectrosc. Relat. Phenom., 2006, 151, 4–8 CrossRef CAS PubMed.
- K. R. J. Lovelock, E. F. Smith, A. Deyko, I. J. Villar-Garcia, P. Licence and R. G. Jones, Chem. Commun., 2007, 4866–4868 RSC.
- A. Deyko and R. G. Jones, Faraday Discuss., 2012, 154, 265–288 RSC.
- S. G. Hessey and R. G. Jones, Chem. Sci., 2013, 4, 2519–2529 RSC.
- R. M. Lynden-Bell and M. Del Popolo, Phys. Chem. Chem. Phys., 2006, 8, 949–954 RSC.
- B. L. Bhargava and S. Balasubramanian, J. Am. Chem. Soc., 2006, 128, 10073–10078 CrossRef CAS PubMed.
- A. S. Pensado, P. Malfreyt and A. A. H. Pádua, J. Phys. Chem. B, 2009, 113, 14708–14718 CrossRef PubMed.
- A. S. Pensado, M. F. Costa Gomes, J. N. Canongia Lopes, P. Malfreyt and A. A. H. Pádua, Phys. Chem. Chem. Phys., 2011, 13, 13518–13526 RSC.
- X. Paredes, J. Fernandez, A. A. H. Pádua, P. Malfreyt, F. Malberg, B. Kirchner and A. S. Pensado, J. Phys. Chem. B, 2012, 116, 14159–14170 CrossRef CAS PubMed.
- X. Paredes, J. Fernandez, A. A. H. Pádua, P. Malfreyt, F. Malberg, B. Kirchner and A. S. Pensado, J. Phys. Chem. B, 2014, 118, 731–742 CrossRef CAS PubMed.
- S. Yockel and G. C. Schatz, J. Phys. Chem. B, 2010, 114, 14241–14248 CrossRef CAS PubMed.
- X. H. Li, G. C. Schatz and D. J. Nesbitt, J. Phys. Chem. B, 2012, 116, 3587–3602 CrossRef CAS PubMed.
- G. Hantal, M. N. D. S. Cordeiro and M. Jorge, Phys. Chem. Chem. Phys., 2011, 13, 21230–21232 RSC.
- G. Hantal, I. Voroshylova, M. Cordeiro and M. Jorge, Phys. Chem. Chem. Phys., 2012, 14, 5200–5213 RSC.
- M. Lísal, Z. Posel and P. Izák, Phys. Chem. Chem. Phys., 2012, 14, 5164–5177 RSC.
- M. Lísal and P. Izák, J. Chem. Phys., 2013, 139, 014704 CrossRef PubMed.
- M. Lísal, J. Chem. Phys., 2013, 139, 214701 CrossRef PubMed.
- W. Jiang, Y. T. Wang, T. Y. Yan and G. A. Voth, J. Phys. Chem. C, 2008, 112, 1132–1139 CAS.
- M. E. Perez-Blanco and E. J. Maginn, J. Phys. Chem. B, 2010, 114, 11827–11837 CrossRef CAS PubMed.
- H. Xu, Z. Han, D. J. Zhang and J. H. Zhan, ACS Appl. Mater. Interfaces, 2012, 4, 6646–6653 CAS.
- H. H. Brongersma, M. Draxler, M. de Ridder and P. Bauer, Surf. Sci. Rep., 2007, 62, 63–109 CrossRef CAS PubMed.
- H. H. Brongersma, in Characterization of materials, ed. E. N. Kaufmann, Wiley, Hoboken, 2nd edn, 2012, pp. 1–23 Search PubMed.
- A. Kauling, G. Ebeling, J. Morais, A. Pádua, T. Grehl, H. H. Brongersma and J. Dupont, Langmuir, 2013, 29, 14301–14306 CrossRef CAS PubMed.
- S. Eminov, J. D. E. T. Wilton-Ely and J. P. Hallett, ACS Sustainable Chem. Eng., 2014, 2, 978–981 CrossRef CAS.
- A. Brandt, M. J. Ray, T. Q. To, D. J. Leak, R. J. Murphy and T. Welton, Green Chem., 2011, 13, 2489–2499 RSC.
- G. Gurau, H. Rodriguez, S. P. Kelley, P. Janiczek, R. S. Kalb and R. D. Rogers, Angew. Chem., Int. Ed., 2011, 50, 12024–12026 CrossRef CAS PubMed.
- L. Chen, M. Sharifzadeh, N. Mac Dowell, T. Welton, N. Shah and J. P. Hallett, Green Chem., 2014, 16, 3098–3106 RSC.
- M. A. Ab Rani, A. Brandt, L. Crowhurst, A. Dolan, N. H. Hassan, J. P. Hallett, P. A. Hunt, M. Lui, H. Niedermeyer, J. M. Perez-Arlandis, M. Schrems, T. Q. To, T. Welton and R. Wilding, Phys. Chem. Chem. Phys., 2011, 13, 16831–16840 RSC.
- H. Tokuda, K. Hayamizu, K. Ishii, M. Abu Bin Hasan Susan and M. Watanabe, J. Phys. Chem. B, 2004, 108, 16593–16600 CrossRef CAS.
- J. G. Huddleston, A. E. Visser, W. M. Reichert, H. D. Willauer, G. A. Broker and R. D. Rogers, Green Chem., 2001, 3, 156–164 RSC.
- M. T. Clough, K. Geyer, P. A. Hunt, J. Mertes and T. Welton, Phys. Chem. Chem. Phys., 2013, 15, 20480–20495 RSC.
- T. Cremer, C. Kolbeck, K. R. J. Lovelock, N. Paape, R. Wölfel, P. S. Schulz, P. Wasserscheid, H. Weber, J. Thar, B. Kirchner, F. Maier and H. P. Steinrück, Chem.–Eur. J., 2010, 16, 9018–9033 CrossRef CAS PubMed.
- J. E. Huheey, E. A. Keiter and R. L. Keiter, Inorganic Chemistry: Principles of Structure and Reactivity, HarperCollins, 4th edn, New York, USA, 1993 Search PubMed.
- F. H. Allen, O. Kennard, D. G. Watson, L. Brammer, A. G. Orpen and R. Taylor, J. Chem. Soc., Perkin Trans. 2, 1987, S1–S19 RSC.
- T. Janssens, C. Huyghebaert, W. Vandervorst, A. Gildenpfennig and H. H. Brongersma, Appl. Surf. Sci., 2003, 203, 30–34 CrossRef.
- H. Wender, L. F. de Oliveira, P. Migowski, A. F. Feil, E. Lissner, M. H. G. Prechtl, S. R. Teixeira and J. Dupont, J. Phys. Chem. C, 2010, 114, 11764–11768 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4sc00640b |
| This journal is © The Royal Society of Chemistry 2014 |