Carbon nanotube–vitrimer composite for facile and efficient photo-welding of epoxy†
Yang
Yang
,
Zhiqiang
Pei
,
Xiqi
Zhang
,
Lei
Tao
,
Yen
Wei
* and
Yan
Ji
*
The Key Laboratory of Bioorganic Phosphorus Chemistry & Chemical Biology, Department of Chemistry, Tsinghua University, Beijing 100084, China. E-mail: jiyan@mail.tsinghua.edu.cn; weiyen@tsinghua.edu.cn
First published on 23rd April 2014
Abstract
Assembling epoxy, one of the most common and widely used thermosets, by welding with remote control is extremely difficult and has not been realized so far, as epoxy cannot melt or be dissolved. Here we present a very simple but highly efficient solution by exploring the photothermal effect of carbon nanotubes (CNTs) to manipulate the transesterification reaction in vitrimers. The carbon nanotube dispersed vitrimer epoxy presented here can be welded by light within seconds or minutes. Moreover, various CNT–vitrimer epoxy materials with different chemical compositions and physical properties can be joined together. Furthermore, transmission welding can be used to weld CNT–vitrimers with other kinds of epoxy or thermoplastic polymers, which is not applicable to welding by direct heating and impossible to realize using the currently available photoweldable covalently cross-linked polymer networks. Additionally, these networks can be efficiently healed by light without the involvement of any glue or sealing agents.
1. Introduction
From air-craft to automobiles, from electronics to biomedical devices, and from office stationary to household appliances, epoxy has been deeply rooted in almost everything in our life as one of the most important covalently cross-linked polymers (thermosets).1 Welding epoxy materials with remote and spatial control is extensively demanded, especially when direct heating is not an option (e.g. in the presence of valuable heat-sensitive components or complex geometries). Light induced welding of plastics is not new, however, this technique has mainly been applied to thermoplastic polymers.2–5 Due to the infusible and insoluble nature of thermosets, the photo-welding of epoxy remains a challenge. The key limitation is that there is no available material that can be welded by remote stimuli other than heat.Recently, polymers based on covalent adaptable networks6–8 were proposed as a new strategy to weld covalently cross-linked polymer networks. Different dynamic covalent networks have been developed in the past decades.9–24 Among all of them, for the fully covalently cross-linked polymer networks, only those based on radical-exchangeable reactions are responsive to light.6–8 For example, disulfide bonds allow efficient photo-reprocessing and healing.25 However, only a limited number of covalently adaptable networks are reported to be capable of photo-welding so far. As far as we know, only three papers presented the results of photo-welding experiments. One exploited photo-responsive disulfide bonds to weld poly(ethylene glycol) hydrogels using UV light.26 A photoinitiator had to be introduced into the swollen hydrogel. In another case, two pieces of newly cut covalently cross-linked network containing trithiocarbonate could be fused together, without the involvement of any solvent, by UV irradiation for several hours.27 In this case, the welding had to be carried out under a nitrogen atmosphere. To solve this problem, thiuram disulfide units were introduced.28 Visible light irradiation for 1 day led to efficient welding. However, such welding has to be done on samples which are freshly cut, otherwise, the radicals necessary for the welding may decompose or diffuse into the sample. In all of these three photo-weldable systems, it is not only their sensitivity to radical termination reactions that affects their long-term reversibility and stability, but also the requirement for specially synthesized monomers which prevent them from being easily incorporated into epoxy and used for industrial-scale production. Additionally, these systems need ultraviolet and visible light, the penetration ability of which is weaker than infrared light.
Here we present a new solution which overcomes all of the above limitations by dispersing carbon nanotubes into “vitrimers”, so that the welding can be achieved with the composite itself and with other types of epoxy within minutes or even seconds using light. The recent creation of “vitrimers” brought forth a novel method to process covalently cross-linked networks at high temperatures due to a reversible transesterification reaction.29–31 By designing vitrimers, we have made moldable liquid crystalline actuators.31 It is well known that CNTs absorb light of almost all wavelengths, generating a large amount of heat.32 This photothermal effect has been extensively explored to kill cancer cells,33–36 actuate mechanical movement,37–41 convert photothermal energy into electrical power,42 and so on. CNTs have also been introduced into thermoplastics to enable photo-welding.43,44 But the photothermal effect of CNTs has not been exploited to activate and manipulate a chemical reaction, nor to weld thermosets or covalently adaptable networks. We assumed and verified here that such heat can be used to locally trigger the transesterification of vitrimers so as to not only weld, but also heal covalently cross-linked epoxy networks using light. Unlike direct heating, light can be switched on or off immediately. As a remote control, it can be used in many occasions when direct heating fails.
Compared to the previously reported photo-weldable covalently cross-linked polymers based on radical-exchangeable reactions,26–28 this new kind of composites have superior photoweldability and a great potential for mass production for various applications. This is firstly because a large variety of CNT–vitrimer type epoxy composites with different mechanical strengths or other physical properties for various purposes can be manufactured, as vitrimers can be synthesized by the widely used epoxy–acid or epoxy–anhydride reaction.45 Numerous epoxy monomers, acids and anhydrides are already commercially available at low cost. A lot of experience in the preparation of epoxy composites can be adapted to the processing of CNT–vitrimer composites. Secondly, this kind of composite is stable and the welding can be repeatedly carried out at any time as required, without the protection of a N2 atmosphere. There are no sensitive radicals involved. Thirdly, the composite can be welded not only with itself, but also with other kinds of epoxy or even other thermoplastic polymers by transmission welding. Light with stronger penetration strength such as infrared light can be used. Many polymers are transparent to infrared light. This enables the utilization of flexible welding techniques such as transmission welding. Finally, the speed of welding is fast. Instead of the hours required by radical-exchange reaction based photo-welding, the welding here takes only minutes or seconds. All of the above are impossible to realize in any available covalently cross-linked polymer networks reported so far.
2. Results and discussion
2.1 Synthes, preparation and thermal properties of CNT–vitrimer networks
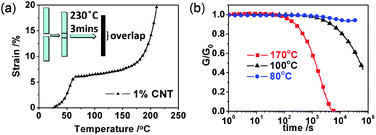
As a demonstration, we synthesized a vitrimer by reacting the diglycidyl ether of bisphenol-A with adipic acid in the presence of triazobicyclodecene as a catalyst for transesterification (Fig. 1a).46 The well-established reaction between di-epoxy and di-carboxyl acid is a complicated process. Once hydroxyl groups are generated by the reaction of epoxy and acid, they continue to react with either epoxy or acid, leading to the branching of polymer chains and the formation of a network.47 Stoichiometric amounts of epoxy and carboxylic acid were used, so that the resulting network contained both hydroxyl and ester groups.29–31 It is well-known that carbon nanotubes are extremely easy to aggregate.48–50 We found that multi-walled CNTs (MWNTs) could be dispersed into the reaction mixture with the help of PIM 1 (a polymer of intrinsic microporosity51). The detailed synthesis and sample preparation of the CNT dispersed vitrimer (CNT–vitrimer) are described in the ESI.† According to a preliminary welding test, the minimum time to obtain a strong weld so that the material snaps at the bulk film instead of the overlapped part during a tensile test was about 3 min, 1 min, 30 s and 30 s for samples with 0.1 wt%, 0.5 wt%, 1 wt% and 3 wt% CNT, respectively. Without CNT, the sample could not be welded at all even after 5 minutes. Since higher concentrations of CNTs will add additional cost for industrial production and change the mechanical properties of the epoxy resin to a larger extent, we chose a CNT concentration of 1 wt% for further detailed investigation. The resulting network was insoluble at all temperatures when swelled in trichlorobenzene. The volume of the sample increased by 21% after 1 h at 100 °C in trichlorobenzene. The volume remained the same after subsequent swelling at 120 °C, 140 °C and 160 °C for 1 h respectively. This proved that the epoxy was covalently cross-linked at all temperatures. The final sample after swelling at 160 °C for 1 h is shown in Fig. 1a. According to Differential Scanning Calorimetry (DSC) (Fig. 1b), the glass transition (Tg) of the CNT–vitrimer was about 39 °C upon cooling and 45 °C upon heating. The viscoelastic properties of the network were characterized using dynamic mechanical analysis (DMA). Fig. 1c shows the dynamic mechanical spectra from −30 to 105 °C. Like a classical thermoset, it exhibited a major relaxation related to Tg and one rubbery plateau with a modulus of about 1.8 MPa. The glassy-state storage modulus was about 1.7 GPa.Dilatometry experiments29 were performed to measure the topology-freezing transition temperature Tv,29–31,46 which is also regarded as a critical transition similar to the Tg of silica.29 Below the Tv, the transesterification is so slow that the vitrimer behaves like a conventional permanently cross-linked polymer network. Above Tv, the transesterification becomes accelerated and the network starts to flow like a viscoelastic fluid but keeps its integrity and number of covalent bonds.29–31 As shown in Fig. 2a, the slope of the strain–temperature curve of the CNT–vitrimer sample increased dramatically after about 160 °C. Since the onset of degradation temperature was above 250 °C according to thermogravimetric analysis (TGA, ESI Fig. S3†), the increase of the slope should not be caused by degradation of the network. Instead, it should be the result of activated transesterification according to ref. 29–31. The appearance of Tv at about 160 °C indicates that the addition of CNTs does not prevent the transesterification, and the composite is malleable at high temperature. The malleability of CNT–vitrimer was tested at 230 °C by a simple compress molding experiment. As seen in the inset of Fig. 2a, two films could be joined together when heated to 230 °C for 3 min under pressure. The rheology experiment is in good agreement with the above result. As shown in Fig. 2b, the shear stress could be released completely at 170 °C (above Tv), indicating that the network flowed at high temperature in spite of the fact that it was covalently cross-linked. On the contrary, it took a much longer time for the sample to relax at temperatures below Tv (e.g. 100 °C and 80 °C).
2.2 Reshaping CNT–vitrimers with light
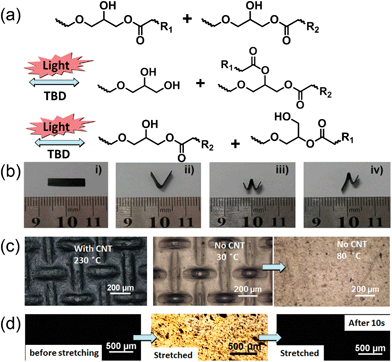
Since the photothermal effect of CNTs can easily increase the local temperature to above 230 °C,52 and we have shown that the transesterification still occurs in the presence of CNTs, we envisioned that light might induce transesterification reactions as depicted in Fig. 3a. When the light irradiation increases the temperature to above Tv, the transesterification is activated, and an ester group and a hydroxyl group react with each other, producing a new ester and a new hydroxyl group which continue to react with other hydroxyl or ester groups to produce hydroxyl and ester groups until a dynamic equilibrium of bond exchange reactions is established. The number of crosslinks and the number of covalent bonds remains the same. Even though almost all wavelengths may work to trigger the transesterification, we used infrared light here. The light triggered transesterification was attested by the reshaping of CNT–vitrimer via an 808 nm laser. As shown in Fig. 3b, irradiation with an IR laser with an intensity of 15.2 W cm−2 for 20 s (the measured temperature was about 180 °C upon such irradiation, which was above Tv) while maintaining the external force converted a straight strip (Fig. 3bi) into a bend (Fig. 3bii), which did not change even when subsequently heated to above 250 °C after the external stress was released. By comparison, for a film without CNTs, the same light irradiation was not able to fix the new shape and the material reverted to its original shape immediately. The bend in Fig. 3biicould be reshaped into a “W” shape (Fig. 3biii) by irradiating the right section at 15.2 W cm−2 (the temperature increased to 180 °C) and the left part at 1.52 W cm−2 (the temperature increased to 55 °C, which was above Tg, but far below Tv) respectively. Therefore, the right part of the “W” was permanently locked while the left part was not. As a result, only the left part recovered its flat shape when the sample was heated to above Tg (Fig. 3biv), as the transesterification was extremely slow at this temperature. The light induced transesterification also provides a new way to “lock” temporary shapes into permanent ones at the microscale. As a representative example, we pressed a film of CNT–vitrimer against a steel woven mesh and simultaneously exposed the set to light (15.2 W cm−2 for 20 s). Upon peeling from the mesh, the film retained the pattern of the steel mesh, which was stable even upon heating to 250 °C (Fig. 3c left panel). However, for the control sample without CNTs, the embossed pattern (Fig. 3c middle panel) vanished immediately when the temperature became higher than Tg (Fig. 3c right panel). Birefringence experiments also confirmed the light induced stress relaxation. Before stretching, the sample under polarized optical microscopy (POM) was completely dark (Fig. 3d left panel). Then it was stretched to 1.5 times its original length. Under POM, the stretched sample was very bright (Fig. 3d middle panel). When the sample was subjected to light, the birefringence disappeared in 10 s because of the relaxation of internal stress due to transesterification (Fig. 3d right panel). The freestanding sample kept its new length when the temperature was raised to more than 240 °C.2.3 Welding by light
The insoluble epoxy network CNT–vitrimer can be efficiently welded by light. As in any welding processes, good contact between two surfaces to be joined together is essential. Thus, we manually pressed two pieces of CNT–vitrimer (10.0 × 1.0 × 0.08 mm) together with an overlap area of 2.0 × 2.0 mm. Then the overlapped part was exposed to IR irradiation (15.2 W cm−2) for 30 s. Even 14 grams of weight (a binder clip) (stress of 34.3 kPa) could not break the welded film (Fig. 4a left panel). To further investigate the welding efficiency, lap-shear tests were carried out on samples welded by light and heat (Fig. 4a right panel). For the joined film which was irradiated for 10 s, the stress at break was ∼5 MPa. A sample welded under the same conditions but with a longer irradiation time (30 s) had a stress at break higher than 20 MPa, and the welded film broke at the bulk materials instead of within the overlapped part. When direct heating was used to weld (180 °C for 10 min), the films could be separated easily by hand under the same conditions. It took at least 10 min to get a relatively strong weld. To further characterize the welding efficiency, we investigated the effect of catalyst concentration and light intensity. Without a catalyst, the sample could not be welded as there was almost no transesterification reaction. As shown in Fig. S4(a),† the weld strength was similar for samples with 5 mol% and 10 mol% catalyst, but the films with 2 mol% catalyst could not be welded well under the same conditions. As for the influence of light intensity, as expected, the stress at break was smaller when lower intensity light was used (Fig. S4(b) in the ESI†).Moreover, the welding can be done specifically on selected areas by inserting a mask between the sample and the light source. For example, as illustrated in Fig. 4b two rectangular masks were placed on top of two overlapped films. When the light was on, only the areas uncovered by the masks were joined together, leaving the rest of the material unaffected. In this way, we obtained a string of two pearls. As another example, we made a pocket out of two films (ESI Fig. S5†). As any epoxy vitrimer synthesized by epoxy–acid or epoxy–anhydride chemistry is capable of the same transesterification, their CNT composites can be welded together by light even when they have different chemical compositions.
Transmission welding makes it possible to join CNT–vitrimers with both epoxy-based and some non-epoxy based polymers. Firstly, CNT–vitrimers can be joined with vitrimers without CNTs. As a demonstration, one sample without CNTs and one with CNTs were pressed together manually. Light was shone on the side of the film without CNTs and was absorbed by the CNTs in the CNT–vitrimer. The two films were joined together after 60 seconds (Fig. 5a). We suppose that heat was generated by the CNTs and spread towards the non-CNT vitrimer, therefore the transesterification occurred at the interfaces of the CNT–vitrimer and non-CNT vitrimer samples. Since vitrimers without CNTs are transparent to IR light, the non-CNT vitrimer could be very thick (ESI Fig. S6†). When a thin layer of CNT–vitrimer was sandwiched between two non-CNT vitrimer layers, those two pieces of non-CNT vitrimers could be joined together when the both sides were subjected to light as if the CNT–vitrimer had been an adhesive (Fig. 5b). This is similar to the photo-adhesive behaviour demonstrated in supramolecular systems,53 but such photo-adhesive behaviour has not been reported in covalently cross-linked networks. Secondly, CNT–vitrimers can be jointed with common epoxy which is cured by diacid or anhydride. Even though common epoxy contains no transesterification catalyst and is not a vitrimer, it can be assembled with CNT–vitrimer as demonstrated in Fig. 5c. We supposed that this was because the catalyst in CNT–vitrimer could migrate to the interface, which made the transesterification between normal epoxy and the vitrimer possible when the light was turned on. Thirdly, CNT–vitrimers can be welded together with thermoplastics. The thermoplastics are weldable when the temperature is around their melting point. As shown in Fig. 5d, the commercially available polyethylene can be easily joined with CNT–vitrimer by IR light due to the photothermal effect of the CNTs in the composite. The welded films in Fig. 5c and d were all inseparable with external force. The welded film snapped in the bulk material, instead of in the welded overlap area. While there is no comparison between the stress–strain curves of pure polymers and the sample which is formed by welding two pieces of films together, we provide this data in the right panel of Fig. 5c and d just to give a rough idea on how strong the welds were.
2.4 Healing with light
The materials reported here can be healed in situ by irradiation in seconds without the addition of a healing agent. Light induced healing is another property that is highly sought-after but challenging to achieve for covalently cross-linked polymer networks. The ability to repair micro-cracks in epoxy networks is of great interest technologically because it could prevent the cracks from developing into a macro-scale failure, and would extend the service lifetime and enhance the safety performance of materials. Usually, covalently cross-linked networks are unable to repair themselves without proper healing agents once a crack develops.54,55 Light induced healing based on photo-crosslinking and photo-triggered metathesis or “reshuffling” reactions can be used for polymers with a low Tg below or around room temperature.7,21,28 Otherwise, the polymers have to be heated to above Tg first so as to have sufficient mobility to diffuse into the damaged area. Recently, photothermal effects have stood out as a new route to trigger the healing of polymers.52,56–60 However, healing by photothermal effect in insoluble polymer networks is rare.59 For the CNT–vitrimers, quick and efficient healing can be achieved by light irradiation even though the network has a Tg well above room temperature. As shown in Fig. 6a, we carved a film with a razor to produce a cut of about 50 μm in width and 5 mm in length. Only 10 s of irradiation (intensity 15.2 W cm−2) by laser light healed the damaged area (Fig. 6a left panel). If the cut was made wider, the cut could still be healed with slightly longer irradiation times. For example, a cut of about 130 μm was healed by IR irradiation for 1 min (Fig. 6a middle panel). The cut healed faster if the sample was covered by a quartz plate which gave rise to some pressure on the film. In another example, the film was pierced by a needle, forming a see-though hole with a diameter of around 140 μm (Fig. 6a right panel). Again, after 5 s of exposure to light, the hole vanished completely. In the control experiments, such cuts in films without CNTs remained even after they were exposed to the same intensity of light for more than 1 hour. Stress–strain experiments on the original and healed films suggested that the mechanical properties were almost the same (Fig. 6b). To our surprise, the healing efficiency by direct heating is quite poor. In Fig. 6c, the width of the cut shrunk from about 62 (Fig. 6c left panel) to 33 μm after 3 min at 180 °C (Fig. 6c middle panel). There was little improvement afterwards, as the cut became 27 μm wide after one hour (Fig. 6c right panel).3. Conclusions
All of the above results have clearly demonstrated that the photo-thermal effect of CNTs is strong enough to activate the transesterification reaction. This makes it possible to weld covalently cross-linked epoxy networks using light, with versatile remote control on the selected areas at any time as required. The welding introduced here exhibits unparalleled advantages in terms of robustness, simplicity, speed and potential for mass production. It is not only CNT–vitrimers of different composition that can be welded together, as CNT–vitrimers can be welded with common epoxy or even thermoplastics. Such photo-modulating welding without glues and molds will be especially suitable for processing complex contours or the in situ joining and repairing of epoxy networks that have been integrated into high value sensitive objects or items, which are crucial for current technologies. Since carbon nanotubes absorb light of almost all wavelengths, a large variety of light sources are available besides IR light. Besides transmission welding, there are still several other kinds of welding techniques utilized in laser welding61 of metals and thermoplastics. Furthermore, carbon nanotubes also convert electric or magnetic energy into heat. With appropriate modification of the composite reported here, electrical and magnetic fields could also be employed to manipulate the welding and healing of epoxy.Acknowledgements
This research was supported by the National Science Foundation of China (no. 21274075 and no. 51203086) and the National 973 Project of China (no. 2011CB935700).Notes and references
- S. W. Goodman, Handbook of Insoluble polymer network Plastics, Cambridge University Press, Cambridge, 2nd edn, 1999 Search PubMed.
- D. Grewell and A. Benatar, Int. Polym. Process., 2007, 22, 43–60 CrossRef CAS.
- D. A. Grewell, A. Benatar and J. B. Park, Plastics and composites welding handbook, Hanser Gardener, Munich, Hanser, Cincinnati, 2003 Search PubMed.
- P. A. Atanasov, Opt. Eng., 1995, 34, 2976–2980 CrossRef CAS PubMed.
- K. Sato, Y. Kurosaki, T. Saito and I. Satoh, Proc. SPIE, 2002, 4637, 528–536 CrossRef CAS PubMed.
- G. L. Fiore, S. J. Rowan and C. Weder, Chem. Soc. Rev., 2013, 42, 7278–7288 RSC.
- D. Habault, H. Zhang and Y. Zhao, Chem. Soc. Rev., 2013, 42, 7244–7256 RSC.
- C. N. Bowman and C. J. Kloxin, Angew. Chem., Int. Ed., 2012, 51, 4272–4274 CrossRef CAS PubMed.
- T. Maeda, H. Otsuka and A. Takahara, Prog. Polym. Sci., 2009, 34, 581–604 CrossRef CAS PubMed.
- R. J. Wojtecki, M. A. Meador and S. J. Rowan, Nat. Mater., 2010, 10, 14–27 CrossRef PubMed.
- S. J. Rowan, S. J. Cantrill, G. R. Cousins, J. K. Sanders and J. F. Stoddart, Angew. Chem., Int. Ed., 2002, 41, 898–952 CrossRef.
- W. G. Skene and J.-M. P. Lehn, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 8270–8275 CrossRef CAS PubMed.
- J.-M. Lehn, Prog. Polym. Sci., 2005, 30, 814–831 CrossRef CAS PubMed.
- G. Deng, C. Tang, F. Li, H. Jiang and Y. Chen, Macromolecules, 2010, 43, 1191–1194 CrossRef CAS.
- P. Zheng and T. J. McCarthy, J. Am. Chem. Soc., 2012, 134, 2024–2027 CrossRef CAS PubMed.
- X. Chen, M. A. Dam, K. Ono, A. Mal, H. Shen, S. R. Nutt, K. Sheran and F. Wudl, Science, 2002, 295, 1698–1702 CrossRef CAS PubMed.
- Y. Zhang, A. A. Broekhuis and F. Picchioni, Macromolecules, 2009, 42, 1906–1912 CrossRef CAS.
- B. J. Adzima, H. A. Aguirre, C. J. Kloxin, T. F. Scott and C. N. Bowman, Macromolecules, 2008, 41, 9112–9117 CrossRef CAS PubMed.
- P. Reutenauer, E. Buhler, P. e. J. Boul, S. e. J. Candau and J. M. Lehn, Chem.–Eur. J., 2009, 15, 1893–1900 CrossRef CAS PubMed.
- Y.-X. Lu, F. o. Tournilhac, L. Leibler and Z. Guan, J. Am. Chem. Soc., 2012, 134, 8424–8427 CrossRef CAS PubMed.
- B. Ghosh and M. W. Urban, Science, 2009, 323, 1458–1460 CrossRef CAS PubMed.
- M. Zhang, D. Xu, X. Yan, J. Chen, S. Dong, B. Zheng and F. Huang, Angew. Chem., 2012, 124, 7117–7121 CrossRef.
- T. F. Scott, A. D. Schneider, W. D. Cook and C. N. Bowman, Science, 2005, 308, 1615–1617 CrossRef CAS PubMed.
- K. Imato, M. Nishihara, T. Kanehara, Y. Amamoto, A. Takahara and H. Otsuka, Angew. Chem., Int. Ed., 2012, 51, 1138–1142 CrossRef CAS PubMed.
- B. T. Michal, C. A. Jaye, E. J. Spencer and S. J. Rowan, ACS Macro Lett., 2013, 2, 694–699 CrossRef CAS.
- B. D. Fairbanks, S. P. Singh, C. N. Bowman and K. S. Anseth, Macromolecules, 2011, 44, 2444–2450 CrossRef CAS PubMed.
- Y. Amamoto, J. Kamada, H. Otsuka, A. Takahara and K. Matyjaszewski, Angew. Chem., 2011, 123, 1698–1701 CrossRef.
- Y. Amamoto, H. Otsuka, A. Takahara and K. Matyjaszewski, Adv. Mater., 2012, 24, 3975–3980 CrossRef CAS PubMed.
- D. Montarnal, M. Capelot, F. Tournilhac and L. Leibler, Science, 2011, 334, 965–968 CrossRef CAS PubMed.
- M. Capelot, D. Montarnal, F. Tournilhac and L. Leibler, J. Am. Chem. Soc., 2012, 134, 7664–7667 CrossRef CAS PubMed.
- Z. Pei, Y. Yang, Q. Chen, E. M. Terentjev, Y. Wei and Y. Ji, Nat. Mater., 2013, 13, 36–41 CrossRef PubMed.
- K. Mizuno, J. Ishii, H. Kishida, Y. Hayamizu, S. Yasuda, D. N. Futaba, M. Yumura and K. Hata, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 6044–6047 CrossRef CAS PubMed.
- R. Singh and S. V. Torti, Adv. Drug Delivery Rev., 2013, 65, 2045–2060 CrossRef CAS PubMed.
- H. K. Moon, S. H. Lee and H. C. Choi, ACS Nano, 2009, 3, 3707–3713 CrossRef CAS PubMed.
- N. W. S. Kam, M. O'Connell, J. A. Wisdom and H. Dai, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 11600–11605 CrossRef CAS PubMed.
- Z. Liu, W. Cai, L. He, N. Nakayama, K. Chen, X. Sun, X. Chen and H. Dai, Nat. Nanotechnol., 2007, 2, 47–52 CrossRef CAS PubMed.
- Y. Ji, Y. Y. Huang, R. Rungsawang and E. M. Terentjev, Adv. Mater., 2010, 22, 3436–3440 CrossRef CAS PubMed.
- M. Behl and A. Lendlein, Mater. Today, 2007, 10, 20–28 CrossRef CAS.
- Q.-Q. Ni, C.-S. Zhang, Y. Fu, G. Dai and T. Kimura, Compos. Struct., 2007, 81, 176–184 CrossRef PubMed.
- J. W. Cho, J. W. Kim, Y. C. Jung and N. S. Goo, Macromol. Rapid Commun., 2005, 26, 412–416 CrossRef CAS.
- Q. Meng, J. Hu and Y. Zhu, J. Appl. Polym. Sci., 2007, 106, 837–848 CrossRef CAS.
- E. Miyako, C. Hosokawa, M. Kojima, M. Yudasaka, R. Funahashi, I. Oishi, Y. Hagihara, M. Shichiri, M. Takashima and K. Nishio, Angew. Chem., 2011, 123, 12474–12478 CrossRef.
- A. Visco, N. Campo, L. Torrisi and F. Caridi, Appl. Phys. A: Mater. Sci. Process., 2011, 103, 439–445 CrossRef CAS.
- E. Rodríguez-Vidal, I. Quintana and C. Gadea, Opt. Laser Technol., 2014, 57, 194–201 CrossRef PubMed.
- C. A. May, Epoxy resins: chemistry and technology, CRC Press, 1988 Search PubMed.
- M. Capelot, M. M. Unterlass, F. Tournilhac and L. Leibler, ACS Macro Lett., 2012, 1, 789–792 CrossRef CAS.
- M. Giamberini, E. Amendola and C. Carfagna, Macromol. Chem. Phys., 1997, 198, 3185–3196 CrossRef CAS.
- N. G. Sahoo, S. Rana, J. W. Cho, L. Li and S. H. Chan, Prog. Polym. Sci., 2010, 35, 837–867 CrossRef CAS PubMed.
- Z. Spitalsky, D. Tasis, K. Papagelis and C. Galiotis, Prog. Polym. Sci., 2010, 35, 357–401 CrossRef CAS PubMed.
- Y. Ji, Y. Y. Huang, A. R. Tajbakhsh and E. M. Terentjev, Langmuir, 2009, 25, 12325–12331 CrossRef CAS PubMed.
- N. B. McKeown and P. M. Budd, Chem. Soc. Rev., 2006, 35, 675–683 RSC.
- R. R. Kohlmeyer, M. Lor and J. Chen, Nano Lett., 2012, 12, 2757–2762 CrossRef CAS PubMed.
- C. Heinzmann, S. Coulibaly, A. Roulin, G. Fiore and C. Weder, ACS Appl. Mater. Interfaces, 2014, 6, 4713–4719 CAS.
- B. Blaiszik, S. Kramer, S. Olugebefola, J. S. Moore, N. R. Sottos and S. R. White, Annu. Rev. Mater. Res., 2010, 40, 179–211 CrossRef CAS.
- H. Jin, C. L. Mangun, D. S. Stradley, J. S. Moore, N. R. Sottos and S. R. White, Polymer, 2012, 53, 581–587 CrossRef CAS PubMed.
- M. Burnworth, L. Tang, J. R. Kumpfer, A. J. Duncan, F. L. Beyer, G. L. Fiore, S. J. Rowan and C. Weder, Nature, 2011, 472, 334–337 CrossRef CAS PubMed.
- L. Huang, N. Yi, Y. Wu, Y. Zhang, Q. Zhang, Y. Huang, Y. Ma and Y. Chen, Adv. Mater., 2013, 25, 2224–2228 CrossRef CAS PubMed.
- J. Fox, J. J. Wie, B. W. Greenland, S. Burattini, W. Hayes, H. M. Colquhoun, M. E. Mackay and S. J. Rowan, J. Am. Chem. Soc., 2012, 134, 5362–5368 CrossRef CAS PubMed.
- H. Zhang and Y. Zhao, ACS Appl. Mater. Interfaces, 2013, 5, 13069–13075 CAS.
- M. V. Biyani, E. J. Foster and C. Weder, ACS Macro Lett., 2013, 2, 236–240 CrossRef CAS.
- W. W. Duley, Laser Welding, Wiley-Interscience, Toronto, 1999 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Sample preparation; thermal properties of CNT–vitrimer; light controlled reshaping, welding and healing. See DOI: 10.1039/c4sc00543k |
| This journal is © The Royal Society of Chemistry 2014 |