Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Uptake of one and two molecules of CO2 by the molybdate dianion: a soluble, molecular oxide model system for carbon dioxide fixation†
Ioana
Knopf
a,
Takashi
Ono
a,
Manuel
Temprado
b,
Daniel
Tofan
a and
Christopher C.
Cummins
*a
aDepartment of Chemistry, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02139-4307, USA
bDepartment of Physical Chemistry, Universidad de Alcalá, Ctra. Madrid-Barcelona Km. 33,600, Madrid 28871, Spain
First published on 5th February 2014
Abstract
Tetrahedral [MoO4]2− readily binds CO2 at room temperature to produce a robust monocarbonate complex, [MoO3(κ2-CO3)]2−, that does not release CO2 even at modestly elevated temperatures (up to 56 °C in solution and 70 °C in the solid state). In the presence of excess carbon dioxide, a second molecule of CO2 binds to afford a pseudo-octahedral dioxo dicarbonate complex, [MoO2(κ2-CO3)2]2−, the first structurally characterized transition-metal dicarbonate complex derived from CO2. The monocarbonate [MoO3(κ2-CO3)]2− reacts with triethylsilane in acetonitrile under an atmosphere of CO2 to produce formate (69% isolated yield) together with silylated molybdate (quantitative conversion to [MoO3(OSiEt3)]−, 50% isolated yield) after 22 hours at 85 °C. This system thus illustrates both the reversible binding of CO2 by a simple transition-metal oxoanion and the ability of the latter molecular metal oxide to facilitate chemical CO2 reduction.
Metal oxide catalysts for CO2 transformations are advantageous based on considerations of cost, ease of re-use, and stability,1 but these advantages come at the expense of our ability to readily characterize such systems at a molecular level of detail. Intrigued by the paucity of soluble transition-metal oxide systems known to react with CO2 in a well-defined manner (e.g., eqn (1)),2–4 we decided to investigate salts of the molybdate dianion in this respect, in order to determine the behavior and mode of reaction (if any) of a simple oxoanion with carbon dioxide as either the potential basis for a new homogeneous catalytic system or as a soluble model for known heterogeneous oxide catalysts. Accordingly, herein we report the finding that molybdate absorbs not just one but two equivalents of CO2 (the second, reversibly) together with complete characterization including single-crystal X-ray diffraction studies of the resulting mono- and dicarbonate complexes.
| LnM=O + CO2 ⇌ LnM(CO3) | (1) |
As our studies were in progress, it was reported that the related tungstate dianion indeed serves as a homogeneous catalyst for CO2 fixation,5 but so far the reaction intermediates in that system have not been isolated. The structural, spectroscopic, and computational details we disclose herein form an excellent point of reference both for understanding tungstate-catalyzed CO2 fixation processes and for developing analogous systems based upon molybdate. Toward the latter goal, we show herein the ability of molybdate to mediate the triethylsilane reduction of CO2 to formate. The present work follows and improves upon our earlier report of titanium trisanilide oxoanion CO2 binding2 in that the present system utilizes essentially non-interacting organic cations (in the earlier system oxophilic alkali-metal cations such as lithium were a necessary ingredient for CO2 uptake) and in that the molybdate dianion is an entirely inorganic species well suited as a discrete, molecular analog of a solid-state metal oxide. Also serving as a precursor to the present work is our report of a cycle for CO2 reduction to CO at a niobium nitride binding site; that system represented our initial foray into ligand-based strategies for CO2 conversion.6
We began our studies with the commercially available sodium molybdate, but soon determined that this organic-media insoluble salt does not react with CO2 under aqueous conditions (as assessed by 95Mo NMR spectroscopy).7 In order to endow the molybdate dianion with solubility in organic media, we prepared [PPN]2[MoO4] (PPN+ = (Ph3P)2N+) in one step from Ag2MoO4 and [PPN]Cl using a modified literature procedure.8 Upon addition of CO2 to a 0.04 M acetonitrile solution of [PPN]2[MoO4] at room temperature, a new species quickly formed. The 95Mo NMR spectrum of the isolated product exhibits a new resonance at +46.7 ppm, no unreacted starting material (+13.2 ppm), but also a small amount of [Mo2O7]2− by-product identified by a signal at −3.8 ppm.9 A new characteristic carbonyl stretch at 1638 cm−1 could also be observed by IR spectroscopy.10
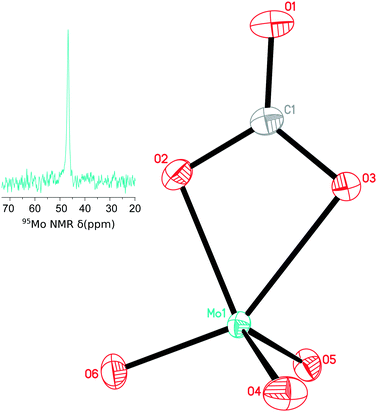
A preliminary X-ray crystal structure of the CO2-addition product revealed a κ2-bound carbonate moiety and enabled us to formulate the major product obtained as [PPN]2[MoO3(κ2-CO3)]. In the interest of obtaining high quality crystallographic data, [NEt4]2[MoO4] (previously reported in the literature and used to prepare examples of well-behaved and crystallographically characterized molybdenum complexes)11 was used to obtain the [MoO3(κ2-CO3)]2− dianion as its tetraethylammonium salt. Colorless crystals were grown by vapor diffusion of Et2O into a CH3CN solution of [NEt4]2[MoO3(κ2-CO3)], and the structure obtained in the ensuing crystallographic investigation is shown in Fig. 1. The C–O distances are elongated from 1.162 Å in free CO212 to 1.2258(13) Å, 1.3048(13) Å, and 1.3357(14) Å in the carbonate unit. The average Mo–O distance is 1.739 Å for the three molybdenum oxo bonds, shorter than the average Mo–O distance of 1.776 Å in tetrahedral [MoO4]2−.13 The carbonate ligand is associated with longer Mo–O interatomic distances at 2.0674(9) and 2.2191(9) Å. The slight asymmetry of the carbonate binding mode is apparently induced by the trans influence of one of the molybdenum oxo ligands (O3–Mo1–O6 148.75(3)° and C1–O3–Mo1–O6 2.09(11)°) as reflected in the Mo–O bond lengths that differ by approximately 0.15 Å, but also in the different Mo–O–C angles of 97.75(6) and 91.74(7)°. This κ2 binding mode is not surprising given the lack of steric bulk around the molybdenum center, in contrast to the arrangement in [(κ1-CO3)TiX3]− (X = N[tBu](3,5-Me2C6H3)) for which a combination of ancillary ligand steric bulk and external carbonate complexation by an alkali-metal counterion promotes κ1-binding of [CO3]2− to the titanium center.2
Solid [PPN]2[MoO3(κ2-CO3)] is moderately stable in air, and does not lose CO2 even after being heated at 70 °C under vacuum for 1 h. In solution, [PPN]2[MoO3(κ2-13CO3)] was heated to 56 °C without any observable loss of 13CO2 as monitored by 13C NMR spectroscopy. However, this compound is moisture sensitive, undergoing conversion to [PPN]2[MoO4] when even a few equivalents of water are added to a solution of [PPN]2[MoO3(κ2-CO3)]. On the other hand, solid [NEt4]2[MoO3(κ2-CO3)] is hygroscopic and converts to molybdate and dimolybdate in ca. 15 minutes by absorbing moisture from the ambient atmosphere, as monitored by IR spectroscopy.
Prepared and isolated using 13CO2, [PPN]2[MoO3(κ2-13CO3)] displays a sharp 13C NMR resonance at 165.7 ppm, this being in a region of the spectrum that is characteristic for carbonates.2,3,14 In its IR spectrum, an isotope-shifted carbonyl stretch is present at 1599 cm−1, in good agreement with the theoretical 1602 cm−1 predicted using the harmonic oscillator approximation. A small peak due to a minor impurity at 158.9 ppm could also be observed by 13C NMR spectroscopy, correlated with the trace dimolybdate by-product detected by 95Mo NMR spectroscopy. Adding [PPN][HCO3]15 to a mixture of [PPN]2[MoO3(κ2-13CO3)] and this unknown species yielded an increase in the intensity of the 158.9 ppm signal and no additional resonances, allowing us to conclusively assign the minor impurity as bicarbonate anion. The minor [HCO3]− impurity may originate from the reaction of [MoO4]2− with [MoO3(κ2-CO3)]2− to yield [Mo2O7]2− and free [CO3]2−, the latter converting to bicarbonate upon protonation, presumably from adventitious water.
Under 1 atm of 13CO2, the room temperature 13C NMR spectrum of a [PPN]2[MoO4] solution features two broad signals: one for the free 13CO2 at 125.8 ppm, and one for the molybdenum carbonate at 165.3 ppm, the broad nature of the resonances suggesting that a chemical exchange is occurring on the NMR time scale. A new major resonance appeared at 162.8 ppm when acquiring the spectrum at −19 °C, but disappeared after degassing the sample. The ratio of the unknown species at 162.8 ppm to [MoO3(κ2-13CO3)]2− increases at higher pressure of 13CO2 (3 atm), and at lower temperature (−31 °C). These data are indicative of additional reversible binding of 13CO2 to the [MoO3(κ2-13CO3)]2− dianion.
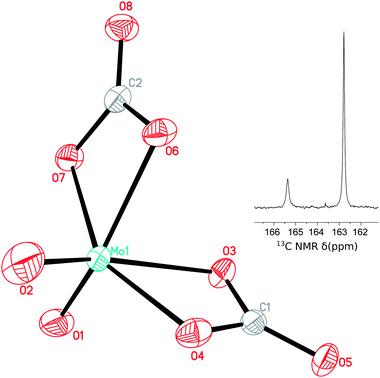
The existence of a dicarbonate species was confirmed by X-ray crystallography, as colorless diffraction quality crystals were grown by slowly cooling a CH3CN solution of [PPN]2[MoO4] under an atmosphere of CO2. In the solid state, both carbonate ligands are bound κ2 (Fig. 2), with Mo–O distances of 2.198(2) and 2.024(2), 2.175(2) and 2.0145(19) Å, respectively. The molybdenum oxo distances are 1.695(2) and 1.705(3) Å, shorter still than in [MoO3(κ2-CO3)]2− as the Mo–O π character is shared over fewer centers. The carbonate ligand binding mode is characterized by the same type of asymmetry as seen in [MoO3(κ2-CO3)]2− due to the trans influence of the oxo ligands (O3–Mo1–O2 152.29(10)°, O6–Mo1–O1 152.10(8)°, C1–O3–Mo1–O2 18.3(2)°, C2–O6–Mo1–O1 27.3(2)°). While we were able to find several examples of κ2-bound molybdenum carbonates reported in the Cambridge Structural Database,16 this is the first example of a molybdenum complex with two κ2-carbonates. To the best of our knowledge, [PPN]2[MoO2(κ2-CO3)2] is also the first transition-metal dicarbonate complex obtained directly from CO2.17
In order to gain further insight into the energetics and potential energy landscape of this system, we turned to computational methods (Fig. 3). Binding of the first CO2 molecule is exothermic and exergonic with a ΔH°(298 K) = −14.2 kcal mol−1 and ΔG°(298 K) = −5.2 kcal mol−1. The stability of the [MoO3(κ2-CO3)]2− species is explained by the considerable activation energy of ΔG‡(298 K) = 17.2 kcal mol−1 for regenerating the molybdate dianion with loss of CO2. This is consistent with our inability to remove CO2 under vacuum at room temperature from this material. As expected, binding of the second CO2 is slightly endergonic (ΔG°(298 K) = 3.2 kcal mol−1), being favored at higher CO2 pressures and lower temperatures as observed in our 13C-labeling experiments. The possibility of binding a third CO2 molecule was also investigated. However, producing such a species is endergonic with a ΔG°(298 K) = 14.6 kcal mol−1, as well as ΔH° > 0 and ΔS° < 0. In contrast to the findings of Mizuno et al. who reported a calculated κ1 structure for the related tungstate–CO2 adduct,5 we were unable to locate minima corresponding to κ1 structures for any of the molybdenum carbonate complexes studied herein.
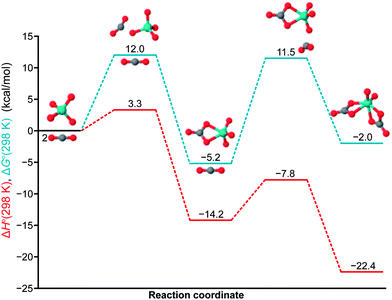 | ||
| Fig. 3 Combined calculated potential energy diagram for the first and second CO2 binding events. Electronic structure calculations were carried out using the M0618 density functional with the Def2-QZVPP19 basis set for molybdenum, incorporating the SDD20 effective core potential, and 6-311+G(3df) for all other atoms as implemented in the Gaussian 09 suite of programs.21 The CPCM model22 for CH3CN was used to describe solvation effects, and the final single-point energies were calculated with QCISD(T)23 at the optimized M06 geometries. | ||
Curious to see whether the new molybdenum carbonate complexes can serve as a source of activated CO2, we subjected [PPN]2[MoO3(κ2-CO3)] to the mild hydride donor triethylsilane, which exhibits no background reactivity with CO2 according to a control experiment.24 A test reaction revealed a new resonance at δ = 8.73 ppm (1H NMR spectroscopy), this being located in a region characteristic for formyl protons, as well as a new molybdenum species having a 95Mo NMR signal at −23.7 ppm, a shift essentially identical to that reported for the [MoO3(OSiMe3)]− anion.9 The production of formate improves from 16% to 71% if the reaction is run under an atmosphere of CO2, raising the question of whether the active species facilitating CO2 reduction is the monocarbonate complex [MoO3(κ2-CO3)]2− or the dicarbonate complex [MoO2(κ2-CO3)2]2−. After optimization, clean conversion to [PPN][OCHO] and [PPN][MoO3(OSiEt3)] (eqn (2)) as the sole products can be obtained after 22 h at 85 °C, as evidenced by the 1H NMR spectrum of the crude reaction mixture. We were able to isolate [PPN][OCHO] in 69% yield, along with [PPN][MoO3(OSiEt3)] in 50% yield by taking advantage of their differential solubilities in THF.
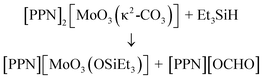 | (2) |
In summary, we have synthesized and characterized two molybdenum oxo carbonate species obtained from the uptake of CO2 by the molybdate dianion and begun exploring their reactivity in the context of CO2 reduction to formate.2513C-labeling experiments and computational analysis suggest that the first binding event to form [MoO3(κ2-CO3)]2− is irreversible, while the second CO2 molecule binds in a reversible process. We are currently investigating potential uses of [MoO4]2− as a nucleophilic catalyst for CO2 fixation26 (encouraged by the methods already developed using the analogous tungstate dianion),5 while also making efforts to develop an improved mechanistic understanding of this rich, yet simple system. This work illustrates the finding that a discrete, soluble molecular metal oxide system unencumbered by organic spectator ligands and unobstructed by hydrogen bonding with water is sufficient for CO2 activation and conversion to formate in conjunction with a mild hydride source.
Acknowledgements
Dr Peter Müller is acknowledged for his invaluable help with the crystal structure refinement of [NEt4]2[MoO3(κ2-CO3)] and [PPN]2[MoO2(κ2-CO3)2]. SABIC (Saudi Basic Industries Corporation) is acknowledged for partly funding the work of IK. TO would like to thank the Spanish Ministry of Education, Culture and Sport (MECD) for financial support. MT acknowledges the Spanish Ministry of Economy and Competitiveness under CTQ2012-36966 for financial support. DT was funded by the National Science Foundation under CHE-1111357. X-ray diffraction data was collected on an instrument purchased with the aid of the National Science Foundation (NSF) under CHE-0946721.References
-
(a) S. Wang, K. Murata, T. Hayakawa, S. Hamakawa and K. Suzuki, Appl. Catal., A, 2000, 196, 1–8 CrossRef CAS
; (b) B. M. Bhanage, S.-I. Fujita, Y. Ikushima and M. Arai, Appl. Catal., A, 2001, 219, 259–266 CrossRef CAS
; (c) M. Matsuoka and M. Anpo, J. Photochem. Photobiol., C, 2003, 3, 225–252 CrossRef CAS
.
- J. S. Silvia and C. C. Cummins, Chem. Sci., 2011, 2, 1474–1479 RSC
.
- J. P. Krogman, M. W. Bezpalko, B. M. Foxman and C. M. Thomas, Inorg. Chem., 2013, 52, 3022–3031 CrossRef CAS PubMed
.
- N. P. Tsvetkov, J. G. Andino, H. Fan, A. Y. Verat and K. G. Caulton, Dalton Trans., 2013, 42, 6745–6755 RSC
.
-
(a) T. Kimura, K. Kamata and N. Mizuno, Angew. Chem., Int. Ed., 2012, 51, 6700–6703 CrossRef CAS PubMed
; (b) T. Kimura, H. Sunaba, K. Kamata and N. Mizuno, Inorg. Chem., 2012, 51, 13001–13008 CrossRef CAS PubMed
.
- J. S. Silvia and C. C. Cummins, J. Am. Chem. Soc., 2010, 132, 2169–2171 CrossRef CAS PubMed
.
- Sodium molybdate dihydrate (50 mg, 0.2 mmol, 1 equiv.) was dissolved in ca. 0.8 mL D2O. Carbon dioxide (20 mL, 0.8 mmol, 4 equiv.) was bubbled through this solution, after which it was stirred for 10 min. The 95Mo NMR spectrum of the resulting solution showed only the molybdate resonance at δ = 0 ppm and no other species.
- J. R. Briggs, A. M. Harrison and J. H. Robson, Polyhedron, 1986, 5, 281–287 CrossRef CAS
.
- Y. Do, E. D. Simhon and R. H. Holm, Inorg. Chem., 1985, 24, 1831–1838 CrossRef CAS
.
- G. Busca and V. Lorenzelli, Mater. Chem., 1982, 7, 89–126 CrossRef CAS
.
-
(a) D. V. Partyka and R. H. Holm, Inorg. Chem., 2004, 43, 8609–8616 CrossRef CAS PubMed
; (b) S. Groysman, J.-J. Wang, R. Tagore, S. C. Lee and R. H. Holm, J. Am. Chem. Soc., 2008, 130, 12794–12807 CrossRef CAS PubMed
.
-
G. Herzberg, Electronic spectra and electronic structure of polyatomic molecules, Van Nostrand, New York, 1966 Search PubMed
.
- P. Román, A. San José, A. Luque, J. M. Gutiérrez-Zorrilla and M. Martínez-Ripoll, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1994, 50, 1189–1191 CrossRef
.
- D. J. Darensbourg, K. M. Sanchez and A. L. Rheingoldib, J. Am. Chem. Soc., 1987, 109, 290–292 CrossRef CAS
.
-
M. L. Meckfessel Jones, Ph.D. thesis, Texas A&M University, 1994
.
-
(a) J. Chatt, M. Kubota, G. J. Leigh, F. C. March, R. Mason and D. J. Yarrow, J. Chem. Soc., Chem. Commun., 1974, 1033–1034 RSC
; (b) E. Carmona, F. Gonzalez, M. L. Poveda, J. M. Marin, J. L. Atwood and R. D. Rogers, J. Am. Chem. Soc., 1983, 105, 3365–3366 CrossRef CAS
; (c) D. M. Curtis and K. R. Han, Inorg. Chem., 1985, 24, 378–382 CrossRef
; (d) K. A. Belmore, R. A. Vanderpool, J.-C. Tsai, M. A. Khan and K. M. Nicholas, J. Am. Chem. Soc., 1988, 110, 2004–2005 CrossRef CAS
; (e) L. Contreras, M. Paneque, M. Sellin, E. Carmona, P. J. Perez, E. Gutierrez-Puebla, A. Monge and C. Ruiz, New J. Chem., 2005, 29, 109–115 RSC
.
- S. V. Krivovichev and P. C. Burns, Radiochemistry, 2004, 46, 12–15 CrossRef CAS
.
- Y. Zhao and D. G. Truhlar, Theor. Chem. Acc., 2008, 120, 215–241 CrossRef CAS
.
- F. Weigend and R. Ahlrichs, Phys. Chem. Chem. Phys., 2005, 7, 3297–3305 RSC
.
- D. Andrae, U. Haeussermann, M. Dolg, H. Stoll and H. Preuss, Theor. Chim. Acta, 1990, 77, 123–141 CrossRef CAS
.
-
M. J. Frisch, et al., Gaussian 09, Revision C.01, 2010 Search PubMed
.
-
(a) V. Barone and M. Cossi, J. Phys. Chem. A, 1998, 102, 1995–2001 CrossRef CAS
; (b) A. Klamt and G. Schuurmann, J. Chem. Soc., Perkin Trans. 2, 1993, 799–805 RSC
.
-
(a) J. A. Pople, M. Head-Gordon and K. Raghavachari, J. Chem. Phys., 1987, 87, 5968–5975 CrossRef CAS PubMed
; (b) P. J. Knowles and H.-J. Werner, Chem. Phys. Lett., 1985, 115, 259–267 CrossRef CAS
.
- Triethylsilane (23 mg, 0.2 mmol) was dissolved in ca. 1 mL CD3CN and transferred to a Schlenk tube which was brought outside the glovebox. The solution was frozen using liquid nitrogen, the headspace was evacuated and the tube refilled with 1 atm of CO2. The tube was sealed and heated at 85 °C overnight. The solution was analyzed by 1H NMR spectroscopy, showing that no reaction had occured.
-
(a) S. Itagaki, K. Yamaguchi and N. Mizuno, J. Mol. Catal. A: Chem., 2013, 366, 347–352 CrossRef CAS PubMed
; (b) W. Sattler and G. Parkin, J. Am. Chem. Soc., 2012, 134, 17462–17465 CrossRef CAS PubMed
; (c) K. Motokura, D. Kashiwame, A. Miyaji and T. Baba, Org. Lett., 2012, 14, 2642–2645 CrossRef CAS PubMed
; (d) R. Lalrempuia, M. Iglesias, V. Polo, P. J. Sanz Miguel, F. J. Fernández-Alvarez, J. J. Pérez-Torrente and L. A. Oro, Angew. Chem., Int. Ed., 2012, 51, 12824–12827 CrossRef CAS PubMed
; (e) A. Jansen and S. Pitter, J. Mol. Catal. A: Chem., 2004, 217, 41–45 CrossRef CAS PubMed
.
-
(a) Y.-B. Wang, Y.-M. Wang, W.-Z. Zhang and X.-B. Lu, J. Am. Chem. Soc., 2013, 135, 11996–12003 CrossRef CAS PubMed
; (b) J. Seayad, A. M. Seayad, J. K. P. Ng and C. L. L. Chai, ChemCatChem, 2012, 4, 774–777 CrossRef CAS
; (c) Z.-Z. Yang, L.-N. He, S.-Y. Peng and A.-H. Liu, Green Chem., 2010, 12, 1850–1854 RSC
; (d) S. N. Riduan, Y. Zhang and J. Y. Ying, Angew. Chem., Int. Ed., 2009, 48, 3322–3325 CrossRef CAS PubMed
; (e) H. Zhou, W.-Z. Zhang, C.-H. Liu, J.-P. Qu and X.-B. Lu, J. Org. Chem., 2008, 73, 8039–8044 CrossRef CAS PubMed
; (f) W. N. Sit, S. M. Ng, K. Y. Kwong and C. P. Lau, J. Org. Chem., 2005, 70, 8583–8586 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Full experimental, crystallographic, spectroscopic, and computational data. CCDC 978136, 978137 and 981116. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4sc00132j |
| This journal is © The Royal Society of Chemistry 2014 |