Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Thermoresponsive organometallic arene ruthenium complexes for tumour targeting†
Catherine M.
Clavel
,
Emilia
Păunescu
,
Patrycja
Nowak-Sliwinska
and
Paul J.
Dyson
*
Institut des Sciences et Ingénierie Chimiques, Ecole Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland. E-mail: paul.dyson@epfl.ch
First published on 2nd January 2014
Abstract
Application of mild hyperthermia can increase the cytotoxicity of anticancer drugs in tumour cells. In this report, we describe low molecular weight thermoactive ruthenium-based drugs with fluorous chains that are selectively triggered by mild hyperthermia. The organometallic complexes were prepared, characterized, and evaluated for their in vitro cytotoxicity against a panel of human cancer cell lines and non-cancerous immortalized cells. The compounds show considerable chemo-thermal selectivity towards cancer cells (ca. 5 μM versus >500 μM for healthy cells) for the compound with the longest fluorous chain.
Introduction
Platinum-based anticancer drugs including cisplatin, carboplatin and oxaliplatin lack selectivity towards cancerous cells and therefore their therapeutic application causes severe side-effects such as nephrotoxicity,1–3 neurotoxicity,4,5 nausea and vomiting.6,7 In contrast, ruthenium-based chemotherapeutics present fewer side-effects compared to platinum-based drugs. Although ruthenium-based compounds are not currently employed in the clinic, two ruthenium(III) compounds, namely KP10198 and NAMI-A,9 completed phase I clinical trials and are currently in phase II trials. The different toxicity profiles of platinum- and ruthenium-based compounds remain unclear, although several reasons have been proposed.10 Irrespective of the full mechanistic differences it is not unreasonable that DNA targeting by platinum compounds leads to the severe side-effects due to the ubiquitous nature of this target. Interestingly, organoruthenium (piano-stool) complexes with the structural composition [RuII(η6-arene)X2(PTA)] (PTA = 1,3,5-triaza-7-phosphaadamantane), known as RAPTA compounds, exhibit anti-metastatic11 and anti-angiogenic12 properties coupled with a relatively low toxicity comparable to that observed for NAMI-A.13In an effort to improve drug selectivity it is possible to enhance the activity of a compound at the tumour site by applying external techniques or inducers.14 One such strategy combines chemotherapy with tumour localised mild hyperthermia.15–17 A slight increase of the local temperature differentiates tissues, healthy ones adapting easily while cancerous cells, with a disorganized and compact vascular structure, have difficulties in dissipating the heat. Some chemotherapeutics exhibit increased activity under mild hyperthermia (40.5–42 °C),18 even though they are also cytotoxic under normal conditions, and were not intentionally designed for this application. The thermosensitivity of small molecule drugs can be enhanced by attaching them to thermoresponsive macromolecules, e.g. liposomal drug carriers19–24 or micelles that are insoluble at 37 °C and become soluble under hyperthermia, enabling them to cross the cell membrane where they release their drug content.25,26 Replacing macromolecules with low molecular weight thermosensitive drugs remains an attractive alternative approach. As proof of concept, rationally designed thermoactive derivatives of the organic drug chlorambucil (CLB)27,28 have been recently designed and were found to be essentially inactive at 37 °C and activated by mild hyperthermia (41 °C) in vitro.29 Recently, the synthesis and biological evaluation (under normal conditions) of some short to medium length fluorous chain bipyridine cisplatin derivatives have been reported.30,31 Similar types of compounds (amphiphilic fluoroalkylated bipyridine platinum and palladium complexes) have also been tested in liposomal formulations.32–34 Liposomal formulations of platinum-based drugs, with the rational that liposomal delivery can increase drug bioavailability and also accumulation at the tumour site as a consequence of the enhanced permeability and retention (EPR) effect, are now in clinical trials.35–38 Herein, ruthenium(II)–arene derivatives (Fig. 1) modified with fluorous chains in order to endow them with thermoresponsive properties39–41 are described.
 | ||
| Fig. 1 Structure of RAPTA-C and the new ruthenium(II)–arene complexes derivatized with alkyl or fluoroalkyl ‘ponytails’. | ||
The general structure of these new ruthenium(II)–arene complexes is similar to that of RAPTA-C (Fig. 1) – the PTA ligand being replaced with the desired fluorous or alkyl derivatized pyridine ligands. The two labile chloride ligands allow activation via hydrolysis following cellular internalization.11,42 Pyridine was selected as the coordinating moiety based on the widespread use of such ligands in the domain.43–51 The fluorous and alkyl chains are connected to the pyridine ligand via an ester linker that may, in principle, be hydrolysed by intracellular enzymes such as esterases.41,52,53
Results and discussion
The proposed approach implies a straightforward synthetic pathway and, consequently, the new derivatives, containing either an alkyl or fluorous chain, were synthesized in two steps using modified pyridine ligands as shown in Scheme 1. The pyridine ligands were obtained in good yield (70–87%) using a standard procedure starting from commercially available 3-pyridine-propionic acid and the corresponding alkyl or fluoroalkyl alcohols. In the second step the pyridine ligands were reacted with the dimer, [Ru(η6-p-cymene)Cl2]2, in anhydrous, degased dichloromethane in the dark under an inert atmosphere. The complexes were isolated by precipitation in good yield (71–87%).All the compounds have been fully characterized (1H, 13C and where appropriate 19F NMR spectroscopy, ESI mass spectrometry, IR spectroscopy and elemental analysis: see ESI for details†). The formation of the ester ligands (both alkyl and perfluoroalkyl derivatives) is accompanied by a deshielding of around 0.4 ppm of the protons in the alpha position relative to the oxygen atom, and subsequent complexation to the ruthenium center via the pyridine N-atom is accompanied by a deshielding of ca. 0.4 ppm for the two pyridine protons in the alpha position to the nitrogen atom and of a deshielding of ca. 5 ppm for the respective carbon atoms. There is only little change in position of the proton signals of the p-cymene ring in comparison to those observed in the parent dimer [Ru(η6-p-cymene)Cl2]2. The structures of the compounds were further corroborated by ESI-MS. The most abundant peaks observed in the spectra of the ligands are those assigned to [M + H]+ ions, whereas the spectra of the pyridine Ru(II)-p-cymene complexes are dominated by species assigned to [M − Cl]+ ions. Apart from the 19F NMR spectra and the very specific 13C NMR profile, the presence of the fluorous chain is also clearly evidenced from the IR spectra with the presence of a strong large peak between 1110 and 1250 cm−1. A peak at ca. 1730 cm−1 confirms the presence of the ester C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group.
O group.
In vitro anticancer activity
The cytotoxicity of the modified pyridine ligands and their corresponding complexes has been assessed in various cancer cell lines (cisplatin-sensitive A2780 and resistant A2780cisR ovarian carcinoma, MCF-7 and MDA-MBA-231 breast carcinomas and A549 human lung carcinoma) and human embryonic kidney (HEK 293) cells (used as a model for normal cells). Cytotoxicity studies were carried out at 37 °C for 72 hours and at 41 °C for 2 hours followed by 70 hours at 37 °C to simulate hyperthermia in the tested cell lines (Table 1).| Compound | A2780 (μM) | A2780cisR (μM) | MCF-7 (μM) | MDA-MB-231 (μM) | A549 (μM) | HEK 293 (μM) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 37 °C | 41 °C | 37 °C | 41 °C | 37 °C | 41 °C | 37 °C | 41 °C | 37 °C | 41 °C | 37 °C | 41 °C | |
| L1a | >500 | 263 ± 12 | >500 | 389 ± 27 | >500 | 458 ± 16 | >500 | 459 ± 27 | 303 ± 17 | 358 ± 20 | >500 | 338 ± 3 |
| L1b | >500 | 98 ± 7 | 224 ± 23 | >500 | >500 | 133 ± 13 | >500 | >500 | >500 | 323 ± 18 | >500 | 153 ± 12 |
| L1c | >500 | 69 ± 4 | 69 ± 4 | 97 ± 15 | 315 ± 48 | 110 ± 7 | 487 ± 9 | >500 | 96 ± 3 | 100 ± 9 | 189 ± 10 | 206 ± 1 |
| L1d | >500 | 40 ± 4 | 88 ± 5 | >500 | 301 ± 75 | >500 | >500 | >500 | >500 | >500 | >500 | >500 |
| L2a | >500 | 181 ± 12 | 141 ± 9 | >500 | 209 ± 5 | 136 ± 1.8 | 362 ± 24 | >500 | 364 ± 8 | >500 | >500 | >500 |
| L2b | 476 ± 164 | 243 ± 35 | 192 ± 7 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 |
| L2c | >500 | >500 | >500 | >500 | 237 ± 25 | 284 ± 31 | >500 | >500 | >500 | >500 | >500 | >500 |
| 1a | >500 | 23 ± 1 | 114 ± 1 | 482 ± 18 | 339 ± 73 | 218 ± 4 | 328 ± 22 | 100 ± 2 | >500 | >500 | 155 ± 17 | 324 ± 10 |
| 1b | >500 | 49 ± 1 | >500 | 362 ± 14 | 319 ± 87 | 108 ± 2 | >500 | 473 ± 20 | >500 | >500 | >500 | 45 ± 3 |
| 1c | 113 ± 2 | 49 ± 1 | 84 ± 1 | 48 ± 1 | >500 | 63 ± 4 | >500 | 96 ± 8 | 391 ± 14 | 123 ± 8 | 86 ± 10 | 42 ± 1 |
| 1d | >500 | 15 ± 1 | >500 | 27 ± 1 | >500 | 17 ± 1 | 70 ± 8 | >500 | >500 | 42 ± 12 | >500 | 149 ± 9 |
| 2a | >500 | 52 ± 2 | 111 ± 1 | >500 | >500 | 70 ± 4 | 275 ± 19 | 67 ± 9 | 355 ± 43 | >500 | 270 ± 18 | 160 ± 6 |
| 2b | 44 ± 1 | 15 ± 1 | 25 ± 2 | 21 ± 1 | 38 ± 2 | 25 ± 2 | 36 ± 2 | 31 ± 2 | 43 ± 1 | 40 ± 2 | >500 | >500 |
| 2c | >500 | 10 ± 1 | >500 | 42 ± 2 | >500 | 5.0 ± 0.3 | >500 | 36 ± 5 | >500 | 33 ± 7 | >500 | 132 ± 5 |
Distinct thermosensitive behaviour of the compounds is present, but needs to be evident against the majority of the tested cancerous cell lines in order to be considered as effective. In this respect, complex 2c exhibits considerable differences of up to at least two orders of magnitude (maximum concentrations tested were 500 μM) and hence exhibits ideal thermoresponsive behaviour. In all cases, complex 2c remains inactive at normal body temperature (IC50 values >500 μM) and becomes toxic towards tumour cells after a 2 hour hyperthermia signal (IC50 values ranging from 5.0 to 42 μM in the various cancer cell lines). Strikingly, the ligand in 2c, i.e.L2c, shows no thermoactivity or cytotoxicity against the screened cell lines except on MCF-7 breast cancer with a negligible (non-thermoresponsive) toxicity of 237 μM at 37 °C and 284 μM under hyperthermia. Moreover, 2c shows selectivity towards cancerous cells with a weak cytotoxicity under mild hyperthermia against HEK 293 cells.
The incorporation of the fluorous chain appears to give rise to the thermoactive effect. Indeed, complex 1c, the hydrocarbon analogue of 2c, exhibits a totally different profile to 2c. It is active in some cell lines at 37 °C and also moderately toxic against non-tumourigenic HEK 293 cells with an IC50 value of 86 μM. Complexes with shorter alkyl chains, i.e.1a and 1b, show generally poor activity against cancerous and non-cancerous cells. Complex 1d, with the longest alkyl chain, exhibits good thermoactivity except in the MDA-MB-231 cell line with an IC50 of 70 μM at 37 °C and >500 μM under hyperthermia. Similarly, its ligand alone, L1d, is only thermoactive in A2780 cells. Indeed, against A2780cisR and MCF-7 cells, L1d is more active than the corresponding complex at normal body temperature, but loses activity under hyperthermia, a behaviour shared with ligands L1b, L2a, L2b and even complex 2a.
Excluding the cisplatin-resistant cell line, only L2a is less cytotoxic under hyperthermia against MDA-MB-231 and A459 cells. Ligands L1d and L2b are inactive at both temperatures in the other cell lines. Ligands with the longest, bulky chains, i.e.L2b, L2c and L1d, are the least active ligands across the panel of cell lines. In A2780 cells the alkylated ligands show increasing cytotoxicity under hyperthermia as the chain length increases, possibly due to increased lipophilicity.
Compounds containing the shorter fluorinated chains do not exhibit a thermoactivity comparable to 2c. Consequently, the length of the fluorous chain has a significant impact on the potential thermoactive behaviour, which is consistent with the results from the study of chlorambucil modified with fluorinated chains.29 Nevertheless, 2b is remarkably cytotoxic and selective towards cancerous cells compared to normal cells, whereas the activity of 2a is not affected by mild hyperthermia in a systematic manner, presumably due to the short fluorous chain.
Cellular uptake
Cellular uptake studies were conducted on the lead complex, i.e.2c, to determine the dependency of uptake on temperature in cancerous and non-cancerous cells. A 2 hour heating at 41 °C was used to simulate the hyperthermia signal during a 24 hour incubation prior to measurement. At 37 °C 2c is internalized three fold more in the A2780 ovarian cancer cell line compared to the normal HEK 293 cells (Fig. 2). Under mild hyperthermia, internalization of 2c in A2780 cells increases whereas heat has little impact on uptake into HEK 293 cells. These data are consistent with the tumour cell selectivity observed for 2c. It should be noted, however, that while uptake of 2c into cancer cells exceeds that in the HEK 293 cells, uptake alone does not explain the vast differences in cytotoxicity following heat treatment. In this context the difficulties cancer cells have dissipating heat54,55 must also make them more susceptible to cell death induced by the internalized compound.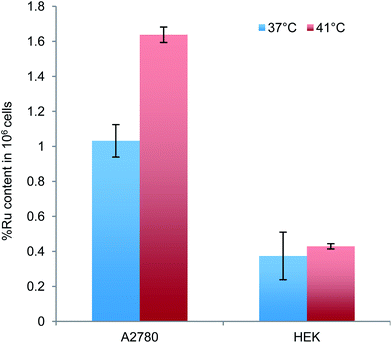 | ||
| Fig. 2 Cellular uptake of 2c in A2780 and HEK 293 cell lines with and without a 2 hour hyperthermia signal at 41 °C. Error bars represent Standard Deviation. | ||
Conclusions
Organometallic ruthenium complexes with a long fluorous appendage exert selective cytotoxicity toward tumour cells under mild hyperthermia. Long fluorous chains are required to obtain relevant thermoresponsive behaviour. For the lead compound, i.e.2c, it is noteworthy that the fluorous ligand alone is not cytotoxic under any of the applied conditions whereas the ruthenium complex demonstrates considerable differences under normal and thermal conditions (ca. 5 μM versus >500 μM) and selectivity towards cancer cells over healthy HEK 293 cells. Discrimination between cancerous and normal cells may be attributed to more extensive internalization by cancer cells compared to normal cells combined with the fact that the tumoural cells are sensitized to the cytotoxic agents under mild hypothermia. This discovery opens the way towards the rational design of other thermoactive anticancer drugs.Acknowledgements
We thank the Swiss National Science Foundation and EPFL for financial support.Notes and references
- X. Yao, K. Panichpisal, N. Kurtzman and K. Nugent, Am. J. Med. Sci., 2007, 334, 115–124 CrossRef PubMed.
- N. Pabla and Z. Dong, Kidney Int., 2008, 73, 994–1007 CrossRef CAS PubMed.
- P. D. Sanchez-Gonzalez, F. J. Lopez-Hernandez, J. M. Lopez-Novoa and A. I. Morales, Crit. Rev. Toxicol., 2011, 41, 803–821 CrossRef CAS PubMed.
- B. Bhhatarai and P. Gramatica, Chem. Res. Toxicol., 2010, 23, 528–539 CrossRef CAS PubMed.
- R. G. van der Hoop, C. J. Vecht, M. E. L. van der Burg, A. Elderson, W. Boogerd, J. J. Heimans, E. P. Vries, J. C. van Houwelingen, F. G. I. Jennekens, W. H. Gispen and J. P. Neijt, N. Engl. J. Med., 1990, 322, 89–94 CrossRef CAS PubMed.
- L. X. Cubeddu, I. S. Hoffmann, N. T. Fuenmayor and A. L. Finn, N. Engl. J. Med., 1990, 322, 810–816 CrossRef CAS PubMed.
- B. Bhhatarai and P. Gramatica, Chem. Res. Toxicol., 2010, 23, 277 CrossRef PubMed.
- C. G. Hartinger, M. A. Jakupec, S. Zorbas-Seifried, M. Groessl, A. Egger, W. Berger, H. Zorbas, P. J. Dyson and B. K. Keppler, Chemistry & Biodiversity, 2008, 5, 2140–2155 CAS.
- J. M. Rademaker-Lakhai, D. van den Bongard, D. Pluim, J. H. Beijnen and J. H. M. Schellens, Clin. Cancer Res., 2004, 10, 3717–3727 CrossRef CAS PubMed.
- C. G. Hartinger and P. J. Dyson, Chem. Soc. Rev., 2009, 38, 391–401 RSC.
- C. Scolaro, A. Bergamo, L. Brescacin, R. Delfino, M. Cocchietto, G. Laurenczy, T. J. Geldbach, G. Sava and P. J. Dyson, J. Med. Chem., 2005, 48, 4161–4171 CrossRef CAS PubMed.
- P. Nowak-Sliwinska, J. R. van Beijnum, A. Casini, A. A. Nazarov, G. Wagnieres, H. van den Bergh, P. J. Dyson and A. W. Griffioen, J. Med. Chem., 2011, 54, 3895–3902 CrossRef CAS PubMed.
- A. Bergamo, C. Gaiddon, J. H. M. Schellens, J. H. Beijnen and G. Sava, J. Inorg. Biochem., 2012, 106, 90–99 CrossRef CAS PubMed.
- N. P. Barry, O. Zava, J. Furrer, P. J. Dyson and B. Therrien, Dalton Trans., 2010, 39, 5272–5277 RSC.
- C. M. Wendtner, S. Abdel-Rahman, M. Krych, J. Baumert, L. H. Lindner, A. Baur, W. Hiddemann and R. D. Issels, J. Clin. Oncol., 2002, 20, 3156–3164 CrossRef CAS PubMed.
- H. I. Robins, J. D. Cohen, C. L. Schmitt, K. D. Tutsch, C. Feierabend, R. Z. Arzoomanian, D. Alberti, F. d'Oleire, W. Longo and C. Heiss, et al. , J. Clin. Oncol., 1993, 11, 1787–1794 CAS.
- J. A. Dickson and S. K. Calderwood, Nature, 1976, 263, 772–774 CrossRef CAS.
- R. D. Issels, Eur. J. Cancer, 2008, 44, 2546–2554 CrossRef CAS PubMed.
- D. Needham, G. Anyarambhatla, G. Kong and M. W. Dewhirst, Cancer Res., 2000, 60, 1197–1201 CAS.
- L. H. Lindner, M. E. Eichhorn, H. Eibl, N. Teichert, M. Schmitt-Sody, R. D. Issels and M. Dellian, Clin. Cancer Res., 2004, 10, 2168–2178 CrossRef CAS.
- L. Li, T. L. M. ten Hagen, D. Schipper, T. M. Wijnberg, G. C. van Rhoon, A. M. M. Eggermont, L. H. Lindner and G. A. Koning, J. Controlled Release, 2010, 143, 274–279 CrossRef CAS PubMed.
- G. Kong, G. Anyarambhatla, W. P. Petros, R. D. Braun, O. M. Colvin, D. Needham and M. W. Dewhirst, Cancer Res., 2000, 60, 6950–6957 CAS.
- A. M. Ponce, Z. Vujaskovic, F. Yuan, D. Needham and M. W. Dewhirst, Int. J. Hyperthermia, 2006, 22, 205–213 CrossRef CAS PubMed.
- S. Unezaki, K. Maruyama, N. Takahashi, M. Koyama, T. Yuda, A. Suginaka and M. Iwatsuru, Pharm. Res., 1994, 11, 1180–1185 CrossRef CAS.
- J. Andrew Mackay and A. Chilkoti, Int. J. Hyperthermia, 2008, 24, 483–495 CrossRef CAS PubMed.
- D. E. Meyer, B. C. Shin, G. A. Kong, M. W. Dewhirst and A. Chilkoti, J. Controlled Release, 2001, 74, 213–224 CrossRef CAS.
- J. E. Ultmann, G. A. Hyman and A. Gellhorn, JAMA, J. Am. Med. Assoc., 1956, 162, 178–183 Search PubMed.
- S. Sachanas, G. A. Pangalis, T. P. Vassilakopoulos, P. Korkolopoulou, F. N. Kontopidou, M. Athanasoulia, X. Yiakoumis, C. Kalpadakis, G. Georgiou, S. Masouridis, M. Moschogiannis, P. Tsirkinidis, V. Pappis, S. I. Kokoris, M. P. Siakantaris, P. Panayiotidis and M. K. Angelopoulou, Leuk. Lymphoma, 2011, 52, 387–393 CrossRef PubMed.
- C. M. Clavel, O. Zava, F. Schmitt, B. H. Kenzaoui, A. A. Nazarov, L. Juillerat-Jeanneret and P. J. Dyson, Angew. Chem., Int. Ed., 2011, 50, 7124–7127 CrossRef CAS PubMed.
- T. T. Chang, S. V. More, N. Lu, J. W. Jhuo, Y. C. Chen, S. C. Jao and W. S. Li, Bioorg. Med. Chem., 2011, 19, 4887–4894 CrossRef CAS PubMed.
- K. E. Elwell, C. Hall, S. Tharkar, Y. Giraud, B. Bennett, C. Bae and S. W. Carper, Bioorg. Med. Chem., 2006, 14, 8692–8700 CrossRef CAS PubMed.
- N. Garelli, P. Vierling, J. L. Fischel and G. Milano, Eur. J. Med. Chem., 1993, 28, 235–242 CrossRef CAS.
- N. Garelli and P. Vierling, Biochim. Biophys. Acta, 1992, 1127, 41–48 CAS.
- N. Garelli and P. Vierling, Inorg. Chim. Acta, 1992, 194, 247–253 CrossRef CAS.
- F. Kratz, I. A. Muller, C. Ryppa and A. Warnecke, ChemMedChem, 2008, 3, 20–53 CrossRef CAS PubMed.
- D. Peer, J. M. Karp, S. Hong, O. C. FaroKHzad, R. Margalit and R. Langer, Nat. Nanotechnol., 2007, 2, 751–760 CrossRef CAS PubMed.
- M. J. Hannon, Pure Appl. Chem., 2007, 79, 2243–2261 CrossRef CAS.
- S. Taurin, H. Nehoff and K. Greish, J. Controlled Release, 2012, 164, 265–275 CrossRef CAS PubMed.
- K. Niikura, K. Nambara, T. Okajima, Y. Matsuo and K. Ijiro, Langmuir, 2010, 26, 9170–9175 CrossRef CAS PubMed.
- F. R. Carrel and P. H. Seeberger, J. Org. Chem., 2008, 73, 2058–2065 CrossRef CAS PubMed.
- B. Hungerhoff, H. Sonnenschein and F. Theil, J. Org. Chem., 2002, 67, 1781–1785 CrossRef CAS PubMed.
- W. H. Ang, E. Daldini, C. Scolaro, R. Scopelliti, L. Juillerat-Jeannerat and P. J. Dyson, Inorg. Chem., 2006, 45, 9006–9013 CrossRef CAS PubMed.
- J. G. Malecki, R. Kruszynski, M. Jaworska, P. Lodowski and Z. Mazurak, J. Organomet. Chem., 2008, 693, 1096–1108 CrossRef CAS PubMed.
- G. Suss-Fink, F. A. Khan, L. Juillerat-Jeanneret, P. J. Dyson and A. K. Renfrew, J. Cluster Sci., 2010, 21, 313–324 CrossRef PubMed.
- J. Grau, V. Noe, C. Ciudad, M. J. Prieto, M. Font-Bardia, T. Calvet and V. Moreno, J. Inorg. Biochem., 2012, 109, 72–81 CrossRef CAS PubMed.
- C. A. Vock, C. Scolaro, A. D. Phillips, R. Scopelliti, G. Sava and P. J. Dyson, J. Med. Chem., 2006, 49, 5552–5561 CrossRef CAS PubMed.
- A. Bacchi, G. Cantoni, M. R. Chierotti, A. Girlando, R. Gobetto, G. Lapadula, P. Pelagatti, A. Sironi and M. Zecchini, CrystEngComm, 2011, 13, 4365–4375 RSC.
- F. Y. Wang, A. Habtemariam, E. P. L. van der Geer, R. Fernandez, M. Melchart, R. J. Deeth, R. Aird, S. Guichard, F. P. A. Fabbiani, P. Lozano-Casal, I. D. H. Oswald, D. I. Jodrell, S. Parsons and P. J. Sadler, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 18269–18274 CrossRef CAS PubMed.
- Y. Fu, A. Habtemariam, A. M. Pizarro, S. H. van Rijt, D. J. Healey, P. A. Cooper, S. D. Shnyder, G. J. Clarkson and P. J. Sadler, J. Med. Chem., 2010, 53, 8192–8196 CrossRef CAS PubMed.
- N. Gligorijevic, S. Arandelovic, L. Filipovic, K. Jakovljevic, R. Jankovic, S. Grguric-Sipka, I. Ivanovic, S. Radulovic and Z. L. Tesic, J. Inorg. Biochem., 2012, 108, 53–61 CrossRef CAS PubMed.
- K. D. Camm, A. El-Sokkary, A. L. Gott, P. G. Stockley, T. Belyaeva and P. C. McGowan, Dalton Trans., 2009, 10914–10925 RSC.
- B. Hungerhoff, H. Sonnenschein and F. Theil, Angew. Chem., Int. Ed., 2001, 40, 2492–2494 CrossRef CAS.
- P. Beier and D. O'Hagan, Chem. Commun., 2002, 1680–1681 RSC.
- C. W. Song, Cancer Res., 1984, 44, 4721–4730 Search PubMed.
- F. K. Storm, W. H. Harrison, R. S. Elliott and D. L. Morton, Cancer Res., 1979, 39, 2245–2251 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed descriptions of the synthesis and characterization of all compounds, procedures for the cytotoxicity determination and cell uptake measurements by ICP-MS. See DOI: 10.1039/c3sc53185f |
| This journal is © The Royal Society of Chemistry 2014 |