Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Transient electronic and vibrational absorption studies of the photo-Claisen and photo-Fries rearrangements†
Stephanie J.
Harris
a,
Daniel
Murdock
a,
Michael P.
Grubb
a,
Gregory M.
Greetham
b,
Ian P.
Clark
b,
Michael
Towrie
b and
Michael N. R.
Ashfold
*a
aSchool of Chemistry, University of Bristol, Bristol, BS8 1TS, UK. E-mail: mike.ashfold@bris.ac.uk
bCentral Laser Facility, Research Complex at Harwell, Science and Technology Facilities Council, Rutherford Appleton Laboratory, Harwell Science and Innovation Campus, Didcot, Oxfordshire OX11 0QX, UK
First published on 27th November 2013
Abstract
The liquid-phase photo-Claisen and photo-Fries rearrangement dynamics of allyl phenyl ether and phenyl acetate in cyclohexane solution have been interrogated via ultrafast transient absorption spectroscopy. Following excitation at 267 nm, the reaction progress is monitored on a picosecond time-scale by electronic and vibrational absorption spectra obtained from broadband UV/Visible and mid-infrared probe pulses. The evolution of the ground and excited electronic states of the parent molecule, the radicals produced by photo-induced homolytic bond fission, and intermediate cyclohexadienones formed via recombination of the produced radical pair are followed, providing new insight and detail on the reaction mechanisms. Subsequent kinetic analysis allows determination of rate coefficients as well as quantum yields for the processes involved. These examples serve to highlight the utility of employing broadband UV-Visible and infrared probe spectroscopies, in conjunction, to unravel the mechanisms of photochemical reactions in solution. The underlying photo-physics that initiates bond fission in this class of molecules is also addressed in the context of the role of dissociative (n/π)σ* excited states.
Introduction
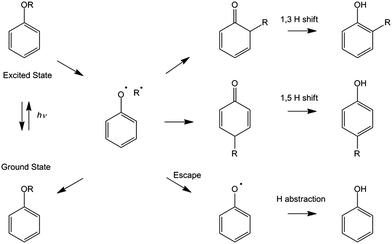
The rearrangements of substituted phenols from either aryl phenyl ethers or phenyl esters into 2- or 4-substituted phenol products are a well-studied class of reactions in synthetic chemistry. First identified by Claisen1 and Fries2,3 in the early 1900's, the thermal Claisen rearrangement results in a 2-substituted phenol produced via a concerted intramolecular reaction whereas a Lewis acid catalyzes the Fries rearrangement and, depending on the experimental conditions, can result in either a 2- or a 4-substituted phenol. Several decades after these initial discoveries, photochemical versions of both rearrangements were uncovered, known as the photo-Claisen4 and the photo-Fries rearrangements.5,6 Subsequent mechanistic studies suggested that UV excitation of the reactant causes homolytic bond fission producing a radical pair which can then recombine to form the original parent molecule. Alternatively, the recombination can occur at either the 2- or 4-positions on the aromatic ring to form a cyclohexadienone intermediate which subsequently undergoes hydrogen atom transfer to form the corresponding substituted phenol as depicted in Scheme 1.7–12 A previous ultrafast study of a 4-substituted phenyl acetate using a UV/Visible probe at several discrete wavelengths by Lochbrunner et al. provided time-resolved support of this mechanism.13The thermal versions of these rearrangements have received extensive attention and evaluation as a key tool in a wide range of syntheses.14–16 With improved understanding and further investigation, the photochemical versions of these rearrangements may offer the same synthetic utility as their thermal counterparts but with the added benefit of being a ‘greener’ synthetic route,17 negating the need for additional reagents (in the case of the Fries rearrangement) and, potentially, requiring reduced energy input. The photo-Fries reaction has previously been used synthetically for the production of compounds including diazoamide macrocycles,18 hydroxyphenones19 and chroman-4-one derivatives.20 A photo-Fries rearrangement has also been identified as the main degradation process of paracetamol following UV excitation.21 The photo-Claisen rearrangement has recently been used in micelle formation.21
This study uses both transient electronic absorption (TEA) and transient vibrational absorption (TVA) spectroscopy to follow the evolution of the species produced by the UV excitation of allyl phenyl ether (PhOC3H5) and phenyl acetate (PhOC(O)CH3) in cyclohexane solution – well-known examples of the photo-Claisen and photo-Fries reactions. As we show below, the combination of transient interrogation by broadband UV/Visible and broadband IR probe pulses reveals a much fuller mechanistic picture of these photo-initiated rearrangements than either technique alone, allowing determination of production and loss rates for various key species and intermediates and estimates of quantum yields – information which, in turn, affords greater understanding of the underlying photophysics driving these processes.
Experimental
The TEA and TVA spectra were recorded using the ULTRA laser facility at the STFC Rutherford Appleton Laboratory.22 An amplified titanium sapphire laser system generated 800 nm (band center) pulses with 50 fs pulse duration at a 10 kHz repetition rate. The broadband UV/Visible probe was generated by focusing a portion of the 800 nm radiation into a CaF2 disc which was rastered in the two planes orthogonal to beam propagation in order to reduce photodamage. A further portion of the 800 nm light was used to pump an optical parametric amplifier to produce mid-IR pulses with a bandwidth of ∼500 cm−1. In each experiment the pump and probe pulses were overlapped in the sample with their respective linear polarization vectors aligned at the magic angle, before the transmitted radiation was dispersed by a grating onto a 512 element silicon array (for UV/Visible detection) or a 128 element mercury cadmium telluride array (for IR detection). The samples were flowed continuously through a Harrick cell with a 100 μm PTFE spacer between CaF2 windows at solution concentrations chosen to ensure an absorbance of 0.5 at 267 nm (30 mM for PhOC3H5 and 180 mM for PhOC(O)CH3). The ∼1 ps experimental response function is limited by background noise induced by the cell windows and solvent (cyclohexane), thus masking the true instrumental response time of ∼100 fs. PhOC3H5 (99%), PhOC(O)CH3 (99%), and cyclohexane were obtained from Sigma-Aldrich and used without further purification.Results
Transient absorption spectroscopy
We begin by considering the TEA spectra of PhOC3H5 and PhOC(O)CH3 in cyclohexane shown in Fig. 1(a) and 2(a) respectively. At the earliest pump-probe delays studied, ∼2 ps, both systems exhibit a broad increase in absorption extending across the full probe region (340–670 nm). In accord with previous aromatic molecules studied via TEA these features can be assigned to absorption from an excited electronic state of the molecule under study.23–25 The temporal evolution of these features are depicted in Fig. 1(c) and 2(d) and are fit well by a single exponential decay which provides a measure of the lifetime of the excited state molecule. A value of 96(4) ps (where the number in parentheses represents the one standard deviation uncertainty in the last significant digit) is obtained for PhOC3H5, while the data for PhOC(O)CH3 reveal an excited state lifetime of 30(3) ps.The TEA spectra of PhOC3H5 and PhOC(O)CH3 exhibit another similarity, a structured feature at 398 nm. This feature has been observed several times previously and is assigned to the phenoxyl radical produced following O–R bond fission.24–26 Due to the substantial overlap of this absorption with that of the excited state parent, the detailed kinetics of the phenoxyl radical cannot be obtained. Homolytic bond fission following UV excitation of PhOC3H5/PhOC(O)CH3 will also produce an allyl/acetyl radical. No absorption of the allyl radical has been reported in the visible probe region studied here, but absorption due to the acetyl radical has previously been recorded in the 490–660 nm range (λmax = 532 nm), with a measured absorption cross section, σ = (1.1 ± 0.2) × 10−19 cm2 per molecule (a molar extinction coefficient, ε, of 29 M−1 cm−1).27 No feature attributable to the acetyl radical is evident at these wavelengths in Fig. 2(a), but this is not unexpected given the weakness of this absorption (cf. for phenoxyl at λmax = 398 nm, σ = 1.2 × 10−17 cm2 per molecule, ε = 3155 M−1 cm−1).26
The TVA spectra recorded following 267 nm photoexcitation of PhOC3H5 and PhOC(O)CH3 are shown in Fig. 1(b), 2(b) and (c), respectively. The spectra of PhOC3H5 show six features within the probe region studied (1400–1700 cm−1), three bleach features reflecting depletion of the ground state population induced by the UV pump pulse at 1495, 1586 and 1598 cm−1 (with strong overlap been the latter two features) and three transient absorptions at 1415, 1516 and 1672 cm−1.
As shown by the kinetic traces in Fig. 1(c) and (d), the transient absorption features at 1415 and 1516 cm−1 show very different kinetic behavior from that exhibited by the feature centered at 1672 cm−1. Fig. 1(c) shows the transient behavior of the feature at 1415 cm−1. It exhibits an exponential decay with a time constant of 108(8) ps. This transient population change suggests that the feature can be assigned to absorption of the excited state of PhOC3H5 as its lifetime is comparable to that of the excited state absorption (ESA) recorded in the TEA spectra; when these two kinetic traces are fit simultaneously to an exponential decay a lifetime of 98(4) ps is obtained. The other transient absorption at 1672 cm−1 shows different kinetics; it is first visible at pump-probe delays of ∼10 ps and grows with an exponential rise time of 150(10) ps. Given this kinetic evolution, it might initially appear tempting to assign this feature to absorption of the phenoxyl radical previously identified in the TEA spectra. This assignment would be incorrect, however, as both previous measurements and calculations do not find or predict any phenoxyl radical absorption in the 1670 cm−1 region.28 Instead, absorptions in this mid-IR wavenumber region are usually associated with a carbonyl group. Two of the proposed intermediates contain a carbonyl group, viz. the substituted 2,4- and 2,5-cyclohexadienones. These are formed when the produced radical pair geminately recombines with, in this case, the aliphatic group rejoining the phenoxyl at either the 2 or the 4 position. However, the predicted carbonyl stretching wavenumbers for these two species are very similar and cannot be distinguished in this experiment (Tables 1 and 2 in the ESI† detail the calculations of the normal modes of PhOC3H5, PhOC(O)CH3 and the substituted 2,4- and 2,5-cyclohexadienone intermediates that have aided these assignments).
Fig. 2(b) and (c) show the TVA spectra obtained for PhOC(O)CH3 with a probe range of 1530–1850 cm−1 and 1810–2000 cm−1, respectively. In this range and over all time delays eight features are discernible, two bleach features at 1604 and 1820 cm−1 and six transient absorption features at 1546, 1557, 1697, 1765, 1803 and 1864 cm−1. Again, the bleaches reflect depopulation of the S0 state of PhOC(O)CH3 by the UV pump pulse. Two of the six transient absorption features, at 1557 and 1803 cm−1, are evident at the earliest pump – probe delays and decay exponentially with a time constant of 28(1) ps. Comparison of this decay constant with those obtained from the analysis of TEA data suggests that these two features correspond to excited state absorption. The same lifetime (28(1) ps) is obtained when the kinetic traces obtained from the TEA and TVA experiments are fit simultaneously.
The feature at 1866 cm−1 exhibits distinctly different kinetic behaviour from the other transient features and is shown in Fig. 2(e). The feature grows for ∼40 ps following excitation, and then begins to decay with some fraction remaining at long time delays. This kinetic behaviour, and the wavenumber at which the band appears, suggests that the feature is due to the acetyl radical produced via O–R bond fission. Previous measurements of the IR spectrum of the acetyl radical in hexane solutions, which found a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching wavenumber of 1864 cm−1, serve to reinforce this assignment.29 Its appearance reflects production by bond fission from the initially excited electronic state of the parent molecule, whereas its subsequent decay is attributable to geminate recombination to form the aforementioned substituted cyclohexadienones or the S0 state parent molecule. The long-time absorption indicates that some fraction of the radical products escapes recombination and persists beyond 2.5 ns in solution.
O stretching wavenumber of 1864 cm−1, serve to reinforce this assignment.29 Its appearance reflects production by bond fission from the initially excited electronic state of the parent molecule, whereas its subsequent decay is attributable to geminate recombination to form the aforementioned substituted cyclohexadienones or the S0 state parent molecule. The long-time absorption indicates that some fraction of the radical products escapes recombination and persists beyond 2.5 ns in solution.
The two features at 1697 and 1765 cm−1 evolve concurrently and, analogous to PhOC3H5, can be ascribed to formation of either or both the substituted 2,5- or 2,4-cyclohexadienone intermediates. The intermediates in this case show two C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch absorptions, as two distinct carbonyl groups are present: the enone, which is part of the six-membered ring (at 1697 cm−1) and the carbonyl of the acetyl group (at 1765 cm−1). These features exhibit an exponential rise of 48(2) ps. As was the case for PhOC3H5, the predicted wavenumbers of the carbonyl stretching modes in the substituted cyclohexadienones are too close to allow them to be differentiated in the present TVA spectra. However, the predicted IR transition strengths (detailed in the ESI†) offer another possible route to discriminating between these two intermediates. The calculated ratio of the enone to acetyl C
O stretch absorptions, as two distinct carbonyl groups are present: the enone, which is part of the six-membered ring (at 1697 cm−1) and the carbonyl of the acetyl group (at 1765 cm−1). These features exhibit an exponential rise of 48(2) ps. As was the case for PhOC3H5, the predicted wavenumbers of the carbonyl stretching modes in the substituted cyclohexadienones are too close to allow them to be differentiated in the present TVA spectra. However, the predicted IR transition strengths (detailed in the ESI†) offer another possible route to discriminating between these two intermediates. The calculated ratio of the enone to acetyl C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch band intensities is 0.98 in the case of the 2-substituted cyclohexadienone, while in the 4-substituted cyclohexadienone it is 2.3. The experimentally observed ratio is 1.9. If the calculated IR intensities are reliable, this would suggest that the ratio of substituted 2,5-cyclohexadienone to 2,4-cyclohexadienone is ∼2
O stretch band intensities is 0.98 in the case of the 2-substituted cyclohexadienone, while in the 4-substituted cyclohexadienone it is 2.3. The experimentally observed ratio is 1.9. If the calculated IR intensities are reliable, this would suggest that the ratio of substituted 2,5-cyclohexadienone to 2,4-cyclohexadienone is ∼2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The previous time resolved study of the photo-Fries rearrangement used a 4-substituted phenyl acetate specifically to exclude possible competition between the two sites of cyclohexadienone formation.13
1. The previous time resolved study of the photo-Fries rearrangement used a 4-substituted phenyl acetate specifically to exclude possible competition between the two sites of cyclohexadienone formation.13
The TVA spectra also allow any repopulation of the ground electronic state of these two molecules to be monitored via the intensity of the ground state bleach features. Fig. 1(d) and 2(f) show that, over the timescale probed, 2.5 ns, ∼30% of the initially excited population of PhOC3H5 returns to the S0 state while, for PhOC(O)CH3, this recovery is ∼54%. When the extracted kinetic traces are fit to an exponential function, good agreement with the rise time for formation of the intermediate cyclohexadienones (170(35) ps and 60(10) ps for PhOC3H5 and PhOC(O)CH3, respectively) is found. We recognize that internal conversion (IC) from the photoexcited state could contribute to the parent bleach recovery at early pump-probe delays, but any such contribution is hard to discern in the present data as the excited state lifetimes in both systems are similar to that observed for geminate recombination. Any contribution from IC is thus neglected in the following kinetic analysis.
Kinetic modelling
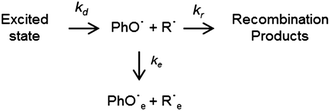
Scheme 2 details the kinetic scheme used to model the processes observed in the photo-Claisen and photo-Fries rearrangements of PhOC3H5 and PhOC(O)CH3, respectively. kd is the rate coefficient for dissociation of the photo-excited molecule to form a radical pair, PhO˙ + R˙. This radical pair can then be lost in two ways; recombination (described by a rate coefficient kr) or escape into the bulk solvent, yielding PhO˙e + R˙e (described by the rate coefficient ke). Recombination can lead to several observable products: S0 parent molecules and the substituted 2,4- and 2,5-cyclohexadienones. An analogous scheme has been used previously to model the kinetics of electron photodetachment from halide anions.30,31Treating the (PhO˙ + R˙) radical pair as a single entity allows analytical solutions for the time dependent concentrations of the four species of interest to be obtained. This treatment has been favoured over one where the geminate recombination process is modelled diffusionally,31 with the assumption that the radical pair is formed instantaneously after UV excitation. Since the lifetimes of the electronically excited parent molecules are in the tens of ps range, such an assumption would be inappropriate in this case. In the case of PhOC(O)CH3, all the transient populations necessary for complete kinetic modelling (viz. the excited and ground electronic states of the parent molecule, the substituted cyclohexadiene, and the C(O)CH3 radical) are accessible. It is important to note that the “in cage” and “bulk” acetyl radicals have the same spectral signature, and the observed signal is consequently the sum of both. Fitting all of the available data, simultaneously, to the kinetic equations detailed in the ESI† yields the rate coefficients and quantum yields shown in Table 1.
| PhOC(O)CH3 | PhOC3H5 | |
|---|---|---|
| a The values with errors in parentheses are obtained or derived from the described kinetic modelling whilst the other quantum yields are estimated from these values. | ||
| k d | 0.0356(8) ps−1 | 0.0102(4) ps−1 |
| k r | 0.034(3) ps−1 | — |
| k e | 0.012(1) ps−1 | — |
| ϕ r | 0.74(8) | 0.86 |
| ϕ S0 | 0.54 | 0.30 |
| ϕ CHD | 0.20 | 0.56 |
| ϕ e | 0.26(8) | 0.14 |
In deriving the latter, we assume an initial quantum yield for dissociation, ϕd = 1. ϕr and ϕe are the respective quantum yields for recombination and escape which can be obtained from the relative magnitudes of ke and kr. The quantum yield for recombination is itself the sum of that for reforming S0 parent molecules, ϕS0, and that for forming the cyclohexadienone adducts, ϕCHD. The percentage ground state bleach recovery provides a measure of ϕS0, with ϕCHD subsequently being obtained from the relation ϕr = ϕS0 + ϕCHD.
Equivalent kinetic analysis for PhOC3H5 is hampered by the lack of transient signal for the C3H5 radical. kd has been acquired from analysis of the transient excited state population, but reliable values for kr and ke are unobtainable. The TEA experiments do, however, provide a relative measure of the long-time phenoxyl radical product yield for both molecules. The above analysis for PhOC(O)CH3 shows that the long-time phenoxyl radical yield (PhO˙e) represents 26% of the originally excited population. Direct comparison of the relative intensities of the long-time phenoxyl absorption at 398 nm thus provides the estimate ϕe = 0.14 in the case of PhOC3H5. Such a comparison is valid as the initial sample concentrations were chosen to ensure the same optical density, 0.5, at the excitation wavelength of 267 nm. Recalling Scheme 2, ϕe = 0.14 implies that the total recombination quantum yield following 267 nm excitation of PhOC3H5 is ϕr = 0.86. As for PhOC(O)CH3, ϕS0 can be obtained from analysis of the ground state bleach recovery, yielding ϕS0 = 0.30 and thus, by difference, ϕCHD = 0.56.
Discussion
The combined use of TEA and TVA techniques has allowed direct confirmation of the mechanism for the first few steps of the photo-Claisen and photo-Fries reactions shown in Scheme 1, while kinetic modeling has provided additional information about the timescales on which these processes occur and their respective quantum yields. Both techniques reveal absorption attributable to the respective excited state molecules and give dissociation lifetimes (in cyclohexane) of 96(4) ps for PhOC3H5 and 28(1) ps for PhOC(O)CH3. Homolytic O–R bond fission is identified as the main population loss channel following photoexcitation. IC to the S0 state cannot be completely ruled out, but is considered a minor process on the basis that the ground state bleach recovery exhibits the same early time kinetics to that of a process (adduct formation) that is unquestionably due to geminate recombination.The tens of picoseconds excited state lifetimes identified in the present study provide a measure of the rate at which population transfers from the initial excited state to one of a dissociative nature. The excited state potential energy landscape relevant to O–R bond fission in a range of aromatic compounds, including PhOC3H5 and PhOC(O)CH3, was explored in an early theoretical paper.32 This study found the S1 state of PhOC3H5 to be a 1ππ* state, the PES of which is intersected by a dissociative 1πσ* state (where the σ* orbital is located along the O–R bond) correlating to PhO + C3H5 products. The acetyl group in PhOC(O)CH3 introduces additional non-bonding and weakly antibonding orbitals. The S1 state in this case was shown to have substantial carbonyl-centered 1nπ* character (i.e. to be formed by removing an electron from an n-orbital on the carbonyl O atom to the π* orbital of the acetyl group), S2 is the ring-centered 1ππ* state and S3 is the dissociative 1πσ* state that forms conical intersections (CIs) with both the 1nπ* and 1ππ* states at short RO–R bond lengths and with the S0 state at longer RO–R. The 1nπ* and 1ππ* states were predicted to lie close in energy, however, and it is not clear which excited state contributes most of the oscillator strength when exciting at 267 nm. These early calculations32 also imply the existence of a barrier to O–R bond dissociation from the S1 potential minimum (PhOC3H5) or from that of the S2 state (PhOC(O)CH3).
Given that UV excitation of both PhOC3H5 and PhOC(O)CH3 results in homolytic O–R bond fission and formation of a radical pair that includes a phenoxyl radical, the well-studied case of phenol (PhOH) is an appropriate point from which to attempt to rationalise the photophysics of PhOC3H5 and PhOC(O)CH3. The potential energy landscape of phenol is qualitatively similar to those exhibited by PhOC3H5 and PhOC(O)CH3. O–H bond fission is observed even when exciting to S1(1ππ*) levels lying at energies below the S1(1ππ*)/S2(1πσ*) CI, i.e. where there is a significant energy barrier to dissociation.33 Time-resolved measurements show the H atom yield rising on a nanosecond timescale,34 which can be understood in terms of O–H bond dissociation by tunneling through the barrier under the S1/S2 CI. At shorter excitation wavelengths (i.e. at energies above the S1/S2 CI), O–H bond fission occurs on a much faster timescale – either as a result of directly populating the dissociative S2 state or following fast radiationless transfer to the S2 PES following initial population of the higher lying S3(1ππ*) state.35 One key difference between the PESs of PhOC3H5, PhOC(O)CH3 and phenol is the difference in bond dissociation energies (i.e. the asymptotic energy associated with radical production). The PhO–H bond strength in phenol is 3.72 eV whereas the PhO–R bond strengths in PhOC3H5 and PhOC(O)CH3 are only 2.16 eV and 3.35 eV, respectively.36 These reduced bond strengths reflect the additional stability of the allyl and acetyl radicals relative to the hydrogen atom produced by phenol photolysis. Recent pump-probe TEA experiments confirm that the same photoexcitation and dissociation dynamics persist for phenol in solution in weakly interacting solvents like cyclohexane though, unsurprisingly, additional post-excitation processes such as geminate recombination of the PhO + H products are also observed.24,25
How then can we reconcile excited state dissociation on a ps timescale following excitation of PhOC3H5 and PhOC(O)CH3? Several points merit note. The topologies of the excited state PESs for PhOC3H5 and PhOC(O)CH3 may be qualitatively similar to that of phenol, but the fragments formed via homolytic O–R bond fission are very different; allyl and acetyl are much larger than an H atom, and tunneling is not a credible fragmentation mechanism. A second consideration is the nature of the level or levels within the excited state manifold that will be populated by absorption of a 267 nm photon. The S1–S0 origin of PhOC3H5 in the gas phase has been identified at 4.50 eV, so absorption of a 267 nm photon (with energy 4.67 eV) will not form PhOC3H5(S1) molecules with substantial internal (vibrational) excitation. Indeed, given that the systems under study are solvated, any such vibrational energy is likely to be quenched by vibrational energy transfer to the surrounding solvent bath. Vibrational cooling in similar systems has been seen to occur on timescales of ∼10 ps (ref. 37) and should thus be competitive for systems such as PhOC3H5 and PhOC(O)CH3 with deduced S1 lifetimes of tens of picoseconds.
Nonetheless, the present data confirm O–R bond fission to be the dominant decay process following 267 nm photoexcitation of both PhOC3H5 and PhOC(O)CH3 as did the previous study of a 4-substituted phenyl acetate,13 and it is pertinent to consider possible limitations of the previous theoretical treatment. The cuts along RO–R in these early PES calculations32 were constrained to Cs geometries (i.e. all the heavy atoms were held in a plane), but a later combined experimental (laser induced fluorescence) and theoretical study of isolated PhOC3H5 identified no fewer than four conformers lying within 500 cm−1 of the planar ground state minimum.38 Recent studies of S–CH3 bond fission in thioanisoles may also be relevant in this regard. As in the phenols, the CI between the bound S1(1ππ*) and dissociative S2(1πσ*) states of thioanisole is calculated to lie at energies above that of the S1(v = 0) level at planar geometries,39 but the barrier to dissociation is seen to decrease upon relaxing the excited state geometry and by changing the C–S–CH3 torsion angle.40 For completeness, we note that the early theoretical study of PhOC(O)CH3 did explore the effect of varying the torsion angle, but found no decrease in the height of the barrier to O–R bond fission.32 New multi-dimensional ab initio calculations on these systems would surely be useful in helping to reconcile the present (and related)13 experimental data. Of course, solvation could also contribute to this apparent mismatch between experiment and theory. Inspection of the available absorption data, presented in the ESI,† shows some red-shift in the peak of the long wavelength UV absorption spectrum of PhOC3H5 in cyclohexane (cf. the gas phase), but the real quantity of interest would be any differential shift in the relative energies of the bound 1ππ*(1nπ*) states and the dissociative 1πσ* PES (since this would affect the height of any barrier under this CI). The low oscillator strength and the diffuseness of the σ* ← π transition precludes its direct observation in absorption measurements.
Post-dissociation, the next step in Scheme 1 determines the fate of the radical pair. This involves competition between two processes: geminate recombination and solvent cage escape. The former leads either to reforming the parent precursor or formation of a substituted cyclohexadienone. As noted above, this substitution may occur at either the 2- or 4-position on the ring (defined relative to the C–O bond) but neither of the present probe techniques allows us to distinguish between these two intermediates. Cage escape leads to a persistent radical signal. In the case of PhOC(O)CH3, the present kinetic modeling suggests that the recombination rate in cyclohexane is ∼3-times faster than the rate of radical cage escape; for PhOC3H5, the relative probability of radical pair recombination is yet greater (∼86%). A more striking difference between the two systems is the relative branching among the recombination products. For PhOC3H5, about two thirds of the recombination events result in substituted cyclohexadienones whereas, for PhOC(O)CH3, the majority recombination product is the S0 parent molecule. A plausible explanation for this difference is that once on the 1πσ* PES, the separating PhO⋯C3H5 products are well posed to recombine in a manner analogous to the concerted reaction observed in the thermal Claisen rearrangement. Such a mechanism should result in preferential production of the substituted 2,4-cyclohexadienone. No such pathway exists for the Fries rearrangement of PhOC(O)CH3, consistent with the deduced lower fractional branching into the adduct in this case.
The final step in Scheme 1 is intramolecular hydrogen transfer within the cyclohexadienone intermediate to form the final substituted phenol product. The present TVA experiments are also capable of probing in the 3000 cm−1 spectral region, where the O–H stretch vibration associated with creation of a phenol product should appear, but no such signal was observed even at the longest pump-probe delays (2.5 ns) – consistent with previous experimental estimates of the timescale on which hydrogen atom transfer occurs in these systems.7,10,13
Conclusions
By using a combination of transient electronic and vibrational absorption spectroscopies, the temporal evolution of the photo-Claisen and photo-Fries rearrangements of PhOC3H5 and PhOC(O)CH3 in cyclohexane solution have been followed for several nanoseconds following photoexcitation at 267 nm. During this time window, the ability to follow both electronic and vibrational absorption of the samples has allowed us to monitor the evolving population of the photoexcited parent molecule and the ensuing O–R bond fission (revealed by the appearance of phenoxyl radical absorption in the TEA experiment and, in the case of PhOC(O)CH3, absorption due to the acetyl radical in the TVA experiment). Subsequent geminate recombination of the resulting radical pair – either to yield a substituted cyclohexadienone intermediate or to reform the parent molecule – is observed also, via growth of new absorption features and ground state bleach recovery in the TVA experiment. The final step in both the photo-Claisen and photo-Fries rearrangements, involving intramolecular hydrogen transfer to transform the cyclohexadienone intermediate into a substituted phenol, is not observed within the time delays probed.Kinetic modelling of the time evolving signals of the various observed species has allowed determination of rate coefficients for each of the above processes, and their relative quantum yields. The fraction of radical pairs formed following photo-excitation that are deduced to escape the initial solvent cage is greater for PhOC(O)CH3 (∼26%) than for PhOC3H5 (14%). Thus most of the radical pairs formed in both cases undergo geminate recombination – but with different outcomes. In the case of PhOC(O)CH3, reforming parent S0 population is favoured over formation of the substituted cyclohexadienone intermediate by a factor of ∼2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, whereas PhOC3H5 shows the opposite behavior. This, we suggest, reflects the fact that the nascent allyl radical arising upon O–R bond fission in the latter case is particularly well posed to add at the 2-position of the ring (as in the thermal Claisen rearrangement).
1, whereas PhOC3H5 shows the opposite behavior. This, we suggest, reflects the fact that the nascent allyl radical arising upon O–R bond fission in the latter case is particularly well posed to add at the 2-position of the ring (as in the thermal Claisen rearrangement).
At a more general level, the present study provides further illustrations of (i) the similarities between the primary UV photochemistry that drives O–R bond fission in these molecules and that responsible for O–H bond fission in simpler systems like phenol, (ii) the ubiquity of πσ* excited states in driving excited state bond fissions in such molecules, and (iii) the utility of detailed gas phase studies of simpler prototypes in guiding our understanding of the, usually more complex, photochemistry of condensed-phase systems for which little gas-phase spectroscopy is available and/or for which high-level theoretical calculations are prohibitive (due to the size of the molecules involved). The present study also highlights the value of broadband transient absorption methods (over the widest possible range of probe wavelengths)41 for elucidating mechanistic aspects of photo-initiated organic reactions in solution – allowing identification of key species, the timescales on which they are created or destroyed and the respective product yields.
Notes and references
- L. Claisen, Chem. Ber., 1912, 45, 3175 CrossRef.
- K. Fries and G. Finck, Chem. Ber., 1908, 41, 4271–4284 CrossRef CAS.
- K. Fries and W. Pfaffendorf, Chem. Ber., 1910, 43, 212–219 CrossRef CAS.
- M. S. Kharesch, G. Stampa and W. Nudenberg, Science, 1952, 116, 309 Search PubMed.
- J. C. Anderson and C. B. Reese, Proc. Chem. Soc., 1960, 217 CAS.
- J. C. Anderson and C. B. Reese, J. Chem. Soc., 1963, 1781–1784 RSC.
- E. Kalmus and D. M. Hercules, J. Am. Chem. Soc., 1972, 96, 449–456 CrossRef.
- S. M. Beck and L. E. Brus, J. Am. Chem. Soc., 1982, 104, 1805–1808 CrossRef CAS.
- A. L. Pincock, J. A. Pincock and R. Stefanova, J. Am. Chem. Soc., 2002, 124, 9768–9778 CrossRef CAS PubMed.
- F. Galindo, J. Photochem. Photobiol., C, 2005, 6, 123–138 CrossRef CAS PubMed.
- I. Iwakura, Phys. Chem. Chem. Phys., 2011, 13, 5546–5555 RSC.
- I. Iwakura, A. Yabushita, J. Liu, K. Okamura and T. Kobayashi, Phys. Chem. Chem. Phys., 2012, 14, 9696–9701 RSC.
- S. Lochbrunner, M. Zissler, J. Piel, E. Riedle, A. Spiegel and T. Bach, J. Chem. Phys., 2004, 120, 11634–11639 CrossRef CAS PubMed.
- F. E. Ziegler, Chem. Rev., 1988, 88, 1423–1452 CrossRef CAS.
- A. M. Martín Castro, Chem. Rev., 2004, 104, 2939–3002 CrossRef PubMed.
- N. Hoffmann, Chem. Rev., 2008, 108, 1052–1103 CrossRef CAS PubMed.
- A. Albini and M. Fagnoni, Green Chem., 2004, 6, 1–6 RSC.
- P. Magnus and C. Lescop, Tetrahedron Lett., 2001, 42, 7193–7196 CrossRef CAS.
- H. Kobsa, J. Org. Chem., 1962, 27, 2293–2298 CrossRef CAS.
- C. S. López, R. Erra-Balsells and S. M. Bonesi, Tetrahedron Lett., 2010, 51, 4387–4390 CrossRef PubMed.
- M. Martignac, E. Oliveros, M.-T. Maurette, C. Claparols and F. Benoit-Marquié, Photochem. Photobiol. Sci., 2013, 12, 527–535 CAS.
- G. M. Greetham, P. Burgos, Q. Cao, I. P. Clark, P. S. Codd, R. C. Farrow, M. W. George, P. Matousek, A. W. Parker, M. R. Pollard, A. Robinson, Z. Xin and M. Towrie, Appl. Spectrosc., 2010, 64, 1311–1319 CrossRef CAS PubMed.
- T. A. A. Oliver, Y. Zhang, M. N. R. Ashfold and S. E. Bradforth, Faraday Discuss., 2011, 150, 439–458 RSC.
- Y. Zhang, T. A. A. Oliver, M. N. R. Ashfold and S. E. Bradforth, Faraday Discuss., 2012, 157, 141–163 RSC.
- S. J. Harris, D. Murdock, Y. Zhang, T. A. A. Oliver, M. P. Grubb, A. J. Orr-Ewing, G. M. Greetham, I. P. Clark, M. Towrie, S. E. Bradforth and M. N. R. Ashfold, Phys. Chem. Chem. Phys., 2013, 15, 6567–6582 RSC.
- X. Chen, D. S. Larsen, S. E. Bradforth and I. H. M. van Stokkum, J. Phys. Chem. A, 2011, 115, 3807–3819 CrossRef CAS PubMed.
- B. Rajakumar, J. E. Flad, T. Gierczak, A. R. Ravishankara and J. B. Burkholder, J. Phys. Chem. A, 2007, 111, 8950–8958 CrossRef CAS PubMed.
- B. M. Giuliano, I. Reva, L. Lapinski and R. Fausto, J. Chem. Phys., 2012, 136, 024505 CrossRef PubMed.
- C. E. Brown, A. G. Neville, D. M. Rayner, K. U. Ingold and J. Lusztyk, Aust. J. Chem., 1995, 48, 363–379 CAS.
- A. Staib and D. Borgis, J. Chem. Phys., 1996, 104, 9027 CrossRef CAS.
- J. A. Kloepfer, V. H. Vilchiz, V. A. Lenchenkov, A. C. Germaine and S. E. Bradforth, J. Chem. Phys., 2000, 113, 6288 CrossRef CAS.
- S. Grimme, Chem. Phys., 1992, 163, 313–330 CrossRef CAS.
- M. G. D. Nix, A. L. Devine, B. Cronin, R. N. Dixon and M. N. R. Ashfold, J. Chem. Phys., 2006, 125, 133318 CrossRef PubMed.
- G. M. Roberts, A. S. Chatterley, J. D. Young and V. G. Stavros, J. Phys. Chem. Lett., 2012, 3, 348–352 CrossRef CAS.
- R. N. Dixon, T. A. A. Oliver and M. N. R. Ashfold, J. Chem. Phys., 2011, 134, 194303 CrossRef PubMed.
- Y.-R. Lao, Handbook of Bond Dissociation Energies in Organic Compounds, 2003 Search PubMed.
- D. Murdock, S. J. Harris, T. N. V. Karsili, G. M. Greetham, I. P. Clark, M. Towrie, A. J. Orr-Ewing and M. N. R. Ashfold, J. Phys. Chem. Lett., 2012, 3, 3715–3720 CrossRef CAS.
- T. Isozaki, T. Suzuki and T. Ichimura, Chem. Phys. Lett., 2007, 449, 63–66 CrossRef CAS PubMed.
- J. S. Lim and S. K. Kim, Nat. Chem., 2010, 2, 627–632 CrossRef CAS PubMed.
- G. M. Roberts, D. J. Hadden, L. T. Bergendahl, A. M. Wenge, S. J. Harris, T. N. V. Karsili, M. N. R. Ashfold, M. J. Paterson and V. G. Stavros, Chem. Sci., 2013, 4, 993–1001 RSC.
- E. Riedle, M. Bradler, M. Wenninger, C. F. Sailer and I. Pugliesi, Faraday Discuss., 2013, 163, 139 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3sc52893f |
| This journal is © The Royal Society of Chemistry 2014 |