Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthetic transporters for sulfate: a new method for the direct detection of lipid bilayer sulfate transport†
Nathalie
Busschaert
a,
Louise E.
Karagiannidis
a,
Marco
Wenzel‡
a,
Cally J. E.
Haynes
a,
Neil J.
Wells
a,
Philip G.
Young
b,
Damjan
Makuc
cd,
Janez
Plavec
*cde,
Katrina A.
Jolliffe
*b and
Philip A.
Gale
*a
aChemistry, University of Southampton, Southampton, SO17 1BJ, UK. Tel: +44 (0)23 8059 3332E-mail: philip.gale@soton.ac.uk
bSchool of Chemistry (F11), The University of Sydney, 2006 NSW, Australia. E-mail: kate.jolliffe@sydney.edu.au; Fax: +61 2 9351 3329; Tel: +61 2 9351 2297
cSlovenian NMR Centre, National Institute of Chemistry, Hajdrihova 19, SI-1000 Ljubljana, Slovenia. E-mail: janez.plavec@ki.si
dEN-FIST Centre of Excellence, Dunajska 156, SI-1000 Ljubljana, Slovenia
eFaculty of Chemistry and Chemical Technology, University of Ljubljana, SI-1000 Ljubljana, Slovenia
First published on 9th January 2014
Abstract
The transmembrane transport of anions by small synthetic molecules is a growing field in supramolecular chemistry and has focussed mainly on the transmembrane transport of chloride. On the other hand, the transport of the highly hydrophilic sulfate anion across lipid bilayers is much less developed, even though the inability to transport sulfate across cellular membranes has been linked to a variety of genetic diseases. Tris-thioureas possess high sulfate affinities and have been shown to be excellent chloride and bicarbonate transporters. Herein we report the sulfate transport abilities of a series of tris-ureas and tris-thioureas based on a tris(2-aminoethyl)amine or cyclopeptide scaffold. We have developed a new technique based on 33S NMR that can be used to monitor sulfate transport, using 33S-labelled sulfate and paramagnetic agents such as Mn2+ and Fe3+ to discriminate between intra- and extravesicular sulfate. Reasonable sulfate transport abilities were found for the reported tris-ureas and tris-thioureas, providing a starting point for the development of more powerful synthetic sulfate transporters that can be used in the treatment of certain channelopathies or as a model for biological sulfate transporters.
Introduction
The lipid bilayer transport of anionic species such as chloride and bicarbonate are key processes in biology mediated by trans-membrane ion channels that regulate membrane potential, cell volume and intracellular pH.1 Synthetic molecules and assemblies that can replicate the transport activity of these natural transport systems either via carrier, relay or channel mechanisms have been reported recently.2 This area is attracting interest as this generation of compounds can replicate the activity of faulty anion channels, and may have future therapeutic potential in the treatment of diseases caused by anion dysregulation (such as cystic fibrosis)3 or in disruption of pH gradients in cells and triggering apoptosis, and hence potential anti-cancer activity.4,5Sulfate transport through lipid bilayers is challenging due to the high hydrophilicity of this anion (ΔGh = −1080 kJ mol−1 as compared to −340 kJ mol−1 for chloride).6 In fact sulfate is often used as a tool in transmembrane transport experiments to determine the transport mechanism as it can usually be assumed that SO42− cannot be transported and hence the use of sulfate as extravesicular anion allows antiport and co-transport processes to be discriminated.7 Although there is some evidence for sulfate transport through synthetic membrane-spanning channels,8 transport of sulfate by mobile carriers that require full or partial dehydration of the anion prior to transport has remained largely unexplored. On the other hand, much effort has been devoted to the problem of selective extraction of sulfate9 from mixtures due to the relevance of this process to nuclear waste remediation.10 A number of groups have also synthesised selective receptors for sulfate in order to meet the challenge of mimicking the selectivity of biological sulfate binding sites.11
From a biological perspective, sulfate is one of the most abundant anions in human plasma and is the major sulfur source in many organisms.12 Sulfate transporters are therefore important biological proteins and misregulation of sulfate transport is present in a number of disease states. For example, defects in the diastrophic dysplasia sulfate transporter (DTDST) can lead to various chondrodysplasia due to insufficient sulfation of cartilage proteoglycans, resulting in symptoms such as dwarfism, spinal deformations and other abnormalities of the joints.13 We therefore decided to examine whether small molecules with high sulfate affinities – that could also encapsulate sulfate within a three dimensional framework shielding it from the hydrophobic interior of the lipid bilayer – could function as transmembrane sulfate transporters. Gale and co-workers have previously reported a series of fluorinated ureas and thioureas based on tris(2-aminoethyl)amine (tren), including compounds 1–6, that showed strong affinity and selectivity for sulfate in DMSO-d6/0.5% water.14,15 It was also observed that compound 4 (and to a lesser extent 3 and 5) were able to transport chloride out of unilamellar phospholipid vesicles in the presence of external sulfate. It was suggested that this was due to the Cl−/SO42− (or HCl/HSO4−) antiport ability of 3–5, but at the time of publication only indirect evidence for this claim could be obtained.
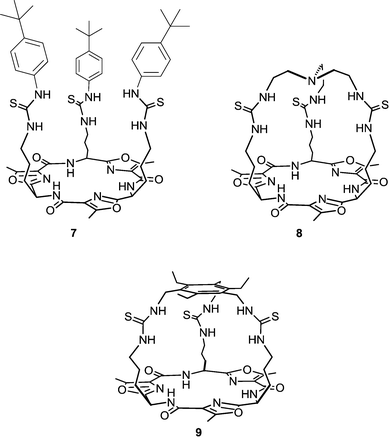
Receptors 1–6 still showed higher transport activity for chloride, nitrate and bicarbonate than for sulfate and we therefore decided to investigate the transmembrane anion transport ability of related compounds with higher sulfate binding affinities. Cage-like anion receptors often have high selectivity for a specific guest and tris-amide cages have previously been used as chloride/nitrate transporters.16 We therefore decided to select potential sulfate transporters from known cage-like sulfate receptors. The recently reported tripodal17 and cryptand-like18,19 anion receptors 7–9, based on a Lissoclinum-type cyclic peptide scaffold, have been found to bind sulfate ions with high affinity (Ka > 104 M−1 in DMSO-d6/0.5% water for all three receptors) and selectivity. This is attributed to the ability of these receptors to form nine hydrogen bonds to sulfate ions – six from the thiourea groups and three from the cyclic peptide backbone amides. In contrast, spherical anions such as chloride bind through the thiourea groups only.17,18 Notably, receptor 8 binds sulfate strongly even in highly competitive solvents (Ka = 1.3 × 103 M−1 in DMSO-d6/20% water at 330 K) indicating that the anion is well shielded from the external environment (compounds 7 and 9 were not soluble enough to allow determination of binding constants under these conditions). In this article we report the anion transport abilities of cyclopeptides 7–9, including the ability of compound 9 to transport the highly hydrophilic sulfate anion across a phospholipid bilayer. We also provide a new method that can directly prove the occurrence of sulfate transport by both the tren-based compounds 1–6 and cyclopeptides 7–9 using 33S NMR.
Results and discussion
Chloride, nitrate and bicarbonate transport by 7–9
The chloride, nitrate and bicarbonate transport abilities of tren-based compounds 1–6 have been previously reported by Gale and co-workers.14,15 It was found that in all cases tris-thioureas (2, 4 and 6) are more potent anion transporters than their analogous tris-ureas (1, 3 and 5) and that the fluorinated transporters (3–6) are more active than their unfluorinated counterparts and have the ability to transport chloride out of phospholipid vesicles at concentrations of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250
250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 carrier-to-lipid (on average one transporter per vesicle).15,20 Gale and co-workers also showed that 1–6 display high affinity for sulfate (Ka > 104 M−1), moderate affinity for chloride (Ka ∼ 500 M−1) and no affinity for nitrate in DMSO-d6/0.5% water. Likewise, Jolliffe and co-workers have found that cyclopeptide based tris-thioureas 7–9 also bind sulfate with high affinity and chloride with moderate affinity (no binding was observed for nitrate).17–19 This similarity in anion binding behaviour between tris-(thio)ureas 1–6 and the structurally related tris-thioureas 7–9, has prompted us to investigate the trans-membrane anion transport ability of cyclopeptides 7–9.
000 carrier-to-lipid (on average one transporter per vesicle).15,20 Gale and co-workers also showed that 1–6 display high affinity for sulfate (Ka > 104 M−1), moderate affinity for chloride (Ka ∼ 500 M−1) and no affinity for nitrate in DMSO-d6/0.5% water. Likewise, Jolliffe and co-workers have found that cyclopeptide based tris-thioureas 7–9 also bind sulfate with high affinity and chloride with moderate affinity (no binding was observed for nitrate).17–19 This similarity in anion binding behaviour between tris-(thio)ureas 1–6 and the structurally related tris-thioureas 7–9, has prompted us to investigate the trans-membrane anion transport ability of cyclopeptides 7–9.
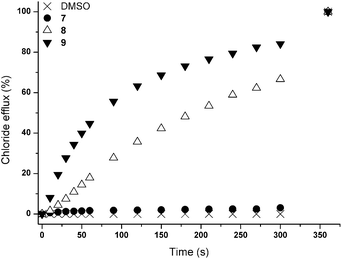
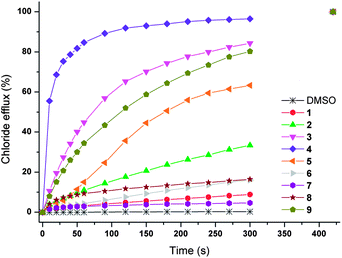
Initially, chloride transport properties were studied using a series of unilamellar 1-palmitoyl-2-oleoylphosphatidylcholine (POPC) vesicles loaded with NaCl (489 mM) and suspended in an external NaNO3 (489 mM) solution using the previously reported procedures utilised for 1–6.15,21 A sample of receptor 7–9 (4 mol% carrier to lipid) was added as a DMSO solution and the resultant chloride efflux was monitored using a chloride selective electrode. After 300 s, the vesicles were lysed by addition of detergent and the final reading of the electrode was used to calibrate 100% release of chloride. The results are shown in Fig. 1 and reveal significant chloride transport by the cryptand-like compounds 8 and 9. The benzyl bridged receptor 9 shows a higher transport activity compared to receptor 8, whilst tripodal receptor 7 was found to be not active. The chloride transport ability of 8 and 9 were further quantified by means of Hill plot,22 where the chloride efflux out of the vesicles is monitored at various concentrations of transporter to yield EC50,270s values (i.e. the concentration of transporter (in mol%) required to obtain 50% chloride efflux in 270 s). The EC50,270s value of compound 9 (1.13 mol%) is three times lower than of compound 8 (3.11 mol%), which points at a substantial influence of the variation in the bridgehead group between the two cryptand-like receptors. Presumably the enhanced lipophilicity of 9 and the larger cavity size, allowing quicker movement of the anions in and out of the cavity (necessary for uptake and release of the anion during transport), result in higher transport activity for receptor 9.
In order to investigate the potential mechanisms of chloride release we repeated the transport experiments with 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 POPC
30 POPC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cholesterol vesicles. The addition of cholesterol decreases the fluidity of the membrane and can therefore have a profound effect on the transport activity of a mobile carrier which relies on diffusion through the bilayer.23 Under these conditions a reduction in chloride transport rate was observed for compounds 8 and 9 (see ESI†), evidence in support of the cyclopeptides functioning as discrete molecular carriers rather than as membrane spanning channels. Furthermore, Hill analyses of transport data reveal n values of 1.1 and 1.0 for 8 and 9 respectively, and also point at a mobile carrier mechanism (see ESI† for Hill plots).24
cholesterol vesicles. The addition of cholesterol decreases the fluidity of the membrane and can therefore have a profound effect on the transport activity of a mobile carrier which relies on diffusion through the bilayer.23 Under these conditions a reduction in chloride transport rate was observed for compounds 8 and 9 (see ESI†), evidence in support of the cyclopeptides functioning as discrete molecular carriers rather than as membrane spanning channels. Furthermore, Hill analyses of transport data reveal n values of 1.1 and 1.0 for 8 and 9 respectively, and also point at a mobile carrier mechanism (see ESI† for Hill plots).24
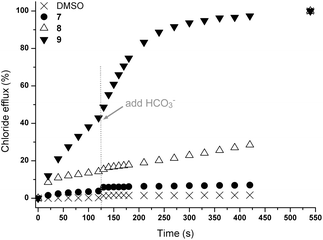
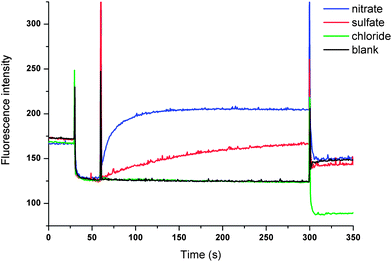
In order to determine whether these results were due to a Cl−/NO3− antiport mechanism, we carried out comparable experiments with sulfate and bicarbonate as external anions. The anions differ in their hydration energy (ΔGhydr(SO42−) −1080 kJ mol−1; ΔGhydr(HCO3−) −335 kJ mol−1; ΔGhydr(NO3−) −300 kJ mol−1)6 and their topology (NO3− and HCO3− planar; SO42− tetrahedral). Consequently an altered transport activity is expected for an antiport mechanism. In this test a series of unilamellar POPC vesicles were prepared loaded with 450 mM NaCl and suspended in an isotonic external Na2SO4 solution (162 mM). Transport is initiated by the addition of a DMSO solution of the receptors (4 mol% with respect to lipid) and two minutes after the addition of the transporters NaHCO3 was added to the external Na2SO4 solution. The results of this combined sulfate and bicarbonate assay are shown in Fig. 2. It can be seen that the addition of NaHCO3 led to a marked acceleration of chloride efflux for receptor 9 while only minor transport is observed for receptor 8, confirming the substantial influence of the bridgehead group on chloride transport (Fig. 2). In addition, a significant chloride efflux was observed in the presence of sulfate as external anion for 9. We have observed such behaviour previously for the open tripodal receptors 1–6 (ref. 15) and this prompted us to investigate the sulfate transport behaviour of these receptors in more detail (vide infra).
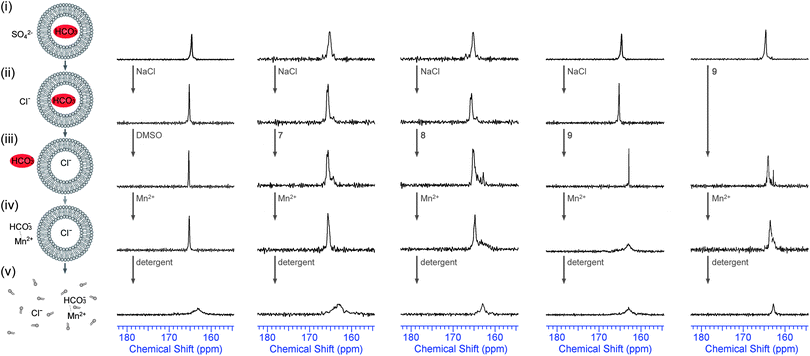
Although the marked difference in transport rate between external nitrate, bicarbonate and sulfate is evidence in support of an antiport mechanism, there is no direct evidence that any anion other than chloride is transported across the phospholipid bilayer by 7–9. In order to provide direct evidence for Cl−/HCO3− antiport, we performed the 13C NMR experiment that was developed by Davis, Gale and Quesada25 and that was also used to prove bicarbonate transport by receptors 1 and 2.14 In these experiments large POPC vesicles (5 μm) were prepared, filled with 13C-labeled NaHCO3 and suspended in an isotonic Na2SO4 solution. One 13C-NMR signal was observed (ca. 165.5 ppm) and this was assigned as the signal for intravesicular H13CO3− (Fig. 3). In order to promote Cl−/HCO3− exchange 100 mM NaCl was added, followed by a DMSO solution of the transporters (4 mol% with respect to lipid) and this mixture was allowed to incubate for 1 hour at room temperature with stirring. The result of this NMR test is shown in Fig. 3 for compounds 7–9 (the results for 1–6 can be found in ESI†). It can be seen that the addition of NaCl did not lead to any leakage of the vesicles as the 13C NMR spectrum remained unchanged. However, when active transporter 9 was added, a new peak appeared 2.5 ppm downfield of the original 13C NMR signal (ca. 163.0 ppm) and this was assigned as the extravesicular H13CO3− signal. In order to prove that this new signal is indeed extravesicular H13CO3− a small amount of paramagnetic Mn2+ was added (1.5 mol% with respect to NaCl). As Mn2+ cannot cross the lipid bilayer, only extravesicular signals are broadened, while intravesicular signals remain sharp. Fig. 3 clearly shows that only cyclopeptide 9 is able to transport HCO3− out of the vesicle, while cyclopeptides 7 and 8 showed no evidence for bicarbonate transport. The combination of this experiment (Fig. 3), where 9 is able to transport HCO3− out of the vesicle in the presence of external chloride, and the previous experiments (Fig. 2), where 9 is able to transport Cl− out of the vesicles in the presence of external bicarbonate, suggests that the receptor 9 functions as a Cl−/HCO3− antiporter. Furthermore, compound 9 is also able to transport HCO3− out of the vesicles when sulfate is the only external anion (no addition of NaCl, Fig. 3 – right hand column), albeit to a smaller extent than in the presence of chloride (again confirming antiport). This suggests that the cyclopeptides can perform the biologically relevant HCO3−/SO42− exchange12 and it gave us another reason to investigate the sulfate transport abilities of these tris-(thio)ureas in more detail.
Indirect evidence for sulfate transport by 1–9
In order to investigate the sulfate transport behaviour of 1–9 in more detail, we started by looking at the chloride efflux mediated by 1–9 (4 mol% carrier to lipid) in the presence of external sulfate. In this test a series of unilamellar POPC vesicles are prepared, loaded with 450 mM NaCl and suspended in an isotonic external Na2SO4 solution (162 mM). Transport is initiated by the addition of a DMSO solution of the receptors (4 mol% with respect to lipid) and chloride efflux is monitored for 5 minutes using a chloride selective electrode. The result of this test is given in Fig. 4 and shows that compounds 3, 4, 5 and 9 show significant chloride transport in the presence of sulfate (>50% chloride efflux in 5 minutes) and are therefore the best candidates for Cl−/SO42− antiport.There are several possible explanations for the chloride transport observed in Fig. 4, including (1) M+/Cl− symport; (2) non-specific leaking or destruction of the vesicles; (3) H+/Cl− symport, OH−/Cl− antiport; (4) Cl−/SO42− antiport. The first option, M+/Cl− symport, was excluded when repeats with encapsulated KCl or CsCl (instead of NaCl) showed no difference in chloride transport and proved that the counter cation does not play a significant role in the chloride transport by 1–9 (see ESI†). The second option, leaking of the vesicles, was discarded using a calcein leakage assay.26 Calcein is a large and highly charged fluorescent dye that self-quenches at high concentration. Thus, when calcein is encapsulated inside vesicles the fluorescence intensity is initially low and increases when it leaks out of the vesicles or when the vesicles are destroyed. However, none of the receptors 1–9 showed any sign of leakage, even after 12 hours (see ESI†). The third alternative explanation, H+/Cl− symport or the equivalent OH−/Cl− antiport, can be investigated by monitoring the internal pH of the vesicle using the pH-sensitive dye 8-hydroxy-1,3,6-pyrenetrisulfonate (HPTS).27 This test has been previously reported for 1–6 and showed either a minor pH increase (1, 2, 3, 6) or a large pH decrease (4, 5). This decrease in pH cannot be explained via an H+/Cl− symport or OH−/Cl− antiport mechanism and it was postulated that the observed pH change could be due to HSO4−/HCl exchange.15 The results for the cyclopeptide-based compounds 7–9 also showed no significant change in the internal pH (see ESI†) and therefore an H+/Cl− symport or OH−/Cl− antiport mechanism can be excluded for all tris-(thio)ureas 1–9 reported in this article.
The only plausible remaining explanation for the transport observed in Fig. 4 is therefore Cl−/SO42− antiport. We have previously developed an indirect method based on the halide sensitive dye lucigenin to provide evidence for sulfate transport.15 This method provided evidence that led us to suggest that compounds 4 and 5 have considerable sulfate transport ability and we now used this method to investigate sulfate transport by compounds 8 and 9. In brief, we prepared a series of vesicles loaded with NaCl and lucigenin (to monitor the intravesicular chloride concentration)28 that were suspended in a NaCl solution (100 mM). The lack of both a chloride and pH gradient rules out HCl co-transport. The experiment was initiated by the addition of various anions (Na2SO4, NaNO3 or NaCl) followed by the addition of a methanol solution of 9. The result depicted in Fig. 5 shows a significant increase in fluorescence upon the addition of 9 when sulfate was added, indicating that chloride is transported out of the vesicle. When NaNO3 was added instead of Na2SO4, a much faster increase in fluorescence was observed, as would be expected for an antiport process of a more lipophilic anion (Fig. 5). As a control, the experiment was repeated with the addition of NaCl instead of Na2SO4. In this case no change in fluorescence was observed, which is evidence in support of an antiport mechanism. No change in fluorescence was observed when compound 8 or methanol was added (see ESI†), indicating that 9 is the only cyclopeptide-based tris-thiourea able to transport sulfate.
Direct evidence of sulfate transport by 1–9 using 33S NMR
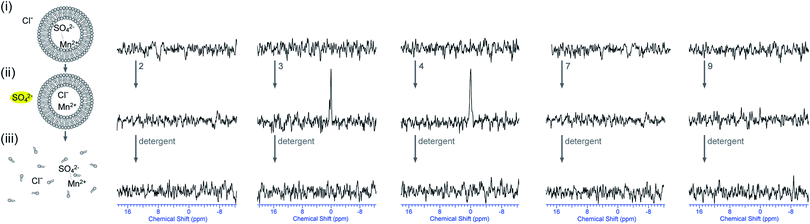
Even though all other possibilities apart from Cl−/SO42− antiport have been excluded, there is still no direct evidence that the highly hydrophilic sulfate anion is transported across the membrane because all tests were conducted by following the movement of chloride. The majority of the biological characterisation of sulfate transporting proteins use 35S radio-labelled sulfate.12,29 However, this method requires the separation of the internal and external solutions prior to measuring radioactive intensities, which can be quite challenging for small dilute vesicles. NMR techniques that can discriminate between the internal and external vesicular environment, without the need for separation, are therefore more suitable and practical for real-time experiments. Thus, we decided to design a method for the direct detection of sulfate transport using 33S NMR techniques similar to the 13C NMR techniques used for the detection of bicarbonate transport in Fig. 3. Due to low natural abundance of 33S, relatively large quadrupole moment and low sensitivity, the use of 33S isotopically labelled sulfate is required for this technique. 33S with its low gyromagnetic ratio exhibits a resonance frequency of 46.0 MHz on a 600 MHz NMR spectrometer. Initially vesicles were prepared containing 33S labelled sodium sulfate and they were dispersed in a sodium chloride solution. Unfortunately, it became apparent that the 33S signal of internal sodium sulfate was at the same 33S chemical shift as that observed in a reference solution of 33Na2SO4 in the absence of vesicles and therefore the position of the peak cannot be used to identify the location of the sulfate anion. Shift reagents are not readily available for sulfate and therefore a different approach was adopted to circumvent this problem, once again using the relaxation properties of paramagnetic Mn2+.In a first set of tests, vesicles were prepared containing 33S labelled Na2SO4 and they were dispersed in a NaCl solution containing 0.5 mol% MnCl2 (with respect to 33SO42−). A small amount of transporter was added as a DMSO solution and the mixture was allowed to incubate for 2 hours at room temperature to allow transport. The results for a selection of compounds are represented in Fig. 6. Before the addition of transporter the 33S NMR spectra consists of a single peak due to internal sulfate. When sulfate is transported out of the vesicle the signal is quenched due to the presence of external Mn2+. As can be seen in Fig. 6, the addition of compounds 4 and 9 leads to a significant reduction in the intensity of the 33S NMR peak, but the peak never disappears into the baseline. The addition of detergent to lyse the vesicles and release all the encapsulated sulfate, however, does lead to a complete disappearance of the 33S NMR signal, indicating that the reduction in intensity was due to incomplete transport rather than insufficient Mn2+ to quench all the sulfate signals. These results seem to suggest that the proposed compounds are indeed able to transport sulfate. However, integration of a single peak without internal reference is not accurate in NMR spectroscopy and we therefore optimised the procedure to obtain more reliable direct evidence of sulfate transport.
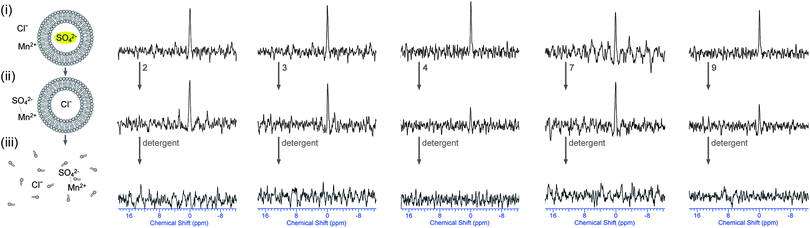
In a second set of experiments, vesicles were prepared containing Na233SO4 and MnSO4 (0.5 mol% Mn2+ with respect to 33SO42−) and were dispersed in a NaCl solution. This way there is no 33S signal prior to the addition of transporter. Addition of transporter could lead to a number of processes: (1) Cl−/SO42− antiport – which would separate the sulfate anions from the Mn2+ ions and would lead to the appearance of a 33S peak; (2) Na+/Cl− symport – which would have no effect on the NMR spectra; (3) Na+ or Mn2+/SO42− symport – which would probably mean that the sulfate signal remains quenched due to the co-transport of manganese; (4) non-specific leaking – where both SO42− and Mn2+ leak out of the vesicle and the signal remains quenched. In other words the appearance of a new 33S NMR peak after incubation with transporter can almost certainly only be due to Cl−/SO42− antiport (or Na+/SO42− co-transport, which is still sulfate transport). The results for a selection of compounds are depicted in Fig. 7 (results of the remaining compounds are in ESI†). It can be seen that the addition of compounds 3 or 4 leads to the appearance of a new 33S NMR signal and for these compounds all of the tests performed indicate sulfate transport. Similarly, the addition of compounds 5 or 6 also led to the appearance of a new 33S signal, while compounds 1, 2 and 7–9 proved to be inactive (see ESI†). Whereas most of these results are consistent with the results observed in Fig. 4, in the case of compound 9 no 33S NMR signal was observed, even after 12 hours incubation with the receptor, whilst all previous tests suggested sulfate transport was mediated by this receptor. Possible reasons for this observation could be the simultaneous transport of sulfate and Mn2+ (or leaking of the vesicles, but this possibility was already ruled out using calcein leakage assays, vide supra). This co-transport may occur due to Mn2+ and SO42− both being doubly charged and hence in the presence of compound 9 forming a neutral molecular ensemble that is lipid soluble.
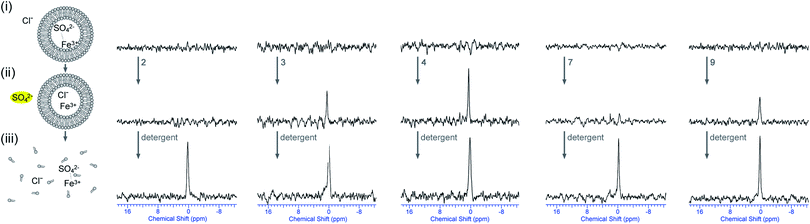
This hypothesis can be tested by repeating the 33S NMR experiments whilst using Fe3+ as the paramagnetic agent. In this case there is a mismatch between the charges of SO42− and Fe3+ and co-transport is therefore less likely. To this end, lipid vesicles were prepared loaded with Na233SO4 and Fe2(SO4)3 and buffered to pH 7.2 using sodium phosphate salts and dispersed in an isotonic NaCl solution. Preliminary tests showed that the addition of 5 mol% Fe3+ (with respect to 33SO42−) was sufficient to broaden the 33S NMR signal, meaning that the initial 33S NMR would show no signal and only the addition of a transporter capable of transporting sulfate would result in a 33S signal. Once detergent is added to lyse the vesicles, it is expected that the 33S NMR would disappear again, proving that the vesicles did not leak during the experiment. Fig. 8 shows the results of these tests where the vesicles were incubated for 2 hours with a selection of compounds (the results of the other compounds can be found in the ESI†). In accordance with the previous tests compounds 3–6 proved to be able to transport sulfate out of the vesicles, while compounds 1, 2, 7 and 8 appeared to be inactive. Receptor 9 on the other hand, which appeared to be inactive when Mn2+ was used as paramagnetic agent, now showed evidence of sulfate transport when Fe3+ was used as paramagnetic agent (Fig. 8). These results suggest that compound 9 is able to transport sulfate via either a Cl−/SO42− antiport process or a Mn2+/SO42− symport process, while compounds 3–6 mainly function via a Cl−/SO42− antiport mechanism and compounds 1, 2, 7 and 8 are unable to transport sulfate. However, Fig. 8 also shows a potential problem when using Fe3+ as paramagnetic agent, namely that the 33S signal increases upon the addition of detergent whereas it was expected to disappear. It was also observed that the addition of detergent led to the formation of a large amount of yellow precipitate, which was identified as iron(III)phosphate using SEM-EDX (see ESI†). It is proposed that disintegration of the vesicles triggers the precipitation of FePO4 (presumably due to excess phosphate buffer upon lysis), thereby removing the majority of paramagnetic Fe3+ out of solution and leading to an increased 33S NMR signal. This is however an advantage, as it clearly shows that the 33S signal appearing upon addition of transporter is due to SO42− being transported and not Fe3+, because the latter would have led to the formation of a FePO4 precipitate prior to the addition of detergent. Therefore, the combination of 33S NMR experiments using Mn2+ or Fe3+ as paramagnetic agents is an excellent way of directly proving sulfate transport, able to discriminate between Cl−/SO42− antiport, Mn+/SO42− symport and indiscriminate leaking of the vesicles.
Table 1 gives an overview of all the tests that were performed on compounds 1–9 and the most likely anion transport mechanisms that can be derived from this information. It can be seen that for the tren-based compounds 1–6, the transporters that are the most potent Cl−/NO3− antiporters (lowest EC50,270s values) are also the most potent bicarbonate and sulfate transporters. This means that these receptors are better at transporting any anion, rather than having an increased selectivity for sulfate. This suggests that the reason why these transporters are better at transporting sulfate is mainly due to the fact that they are more able to screen any hydrophilic anion from the lipid environment (increased lipophilicity, see log P values) and not because they possess increased sulfate affinities. The data in Table 1 shows that it is mainly the lipophilic receptors 3–5 that are able to transport sulfate across lipid bilayers, usually via an antiport mechanism (Cl−/SO42− or HCO3−/SO42−), which is unsurprising as there are many reports about the importance of lipophilicity in anion transport.30 It can also be seen that the data obtained using a chloride selective electrode corresponds well to the 33S NMR data, i.e. sulfate transport can only be observed using 33S NMR when the chloride efflux in the presence of external sulfate is higher than 50% efflux in 300 seconds. The only exception is compound 6, where the sulfate transport ability is lower than initially expected from log P. It has previously been suggested that this receptor is too lipophilic (log P > 11) and therefore it can get “stuck” in the inner layer of the membrane and is not able to pick up any anion from the aqueous phase or the interphase.15 This is probably why the bicarbonate and sulfate transporting abilities of this receptor are impaired.
| C log P | EC50,270sa (Cl−/NO3−) | 13C NMRb (Cl−/HCO3−) | 13C NMRc (SO42−/HCO3−) | HPTSd (ΔpH) | Calceine (leakage) | % Cl− effluxf (Cl−/SO42−) | 33S NMRg (Mn2+) | 33S NMRh (Fe3+) | Proposed transport mechanisms | |
|---|---|---|---|---|---|---|---|---|---|---|
| a EC50,270s = concentration (in mol% carrier to lipid) needed to obtain 50% chloride efflux in 270 s, obtained through Hill plot using vesicles containing NaCl dispersed in a NaNO3 solution (see Fig. 1 and ESI†). b Evidence for HCO3− transport in the presence of external NaCl (yes/no or partial transport) using 13C NMR techniques (see Fig. 3 and ESI†). c Evidence for HCO3− transport in the presence of external Na2SO4 (yes/no or partial transport) using 13C NMR techniques (see Fig. 3 and ESI†). d The magnitude of the intravesicular pH change upon the addition of transporter, determined using vesicles containing NaCl and HPTS and dispersed in a Na2SO4 solution (see ref. 15 and ESI†). e Evidence for leakage of the vesicles (yes or no) using a calcein leakage assay (see ESI†). f Amount of chloride efflux (in %) observed 300 seconds after the addition of 4 mol% of transporter (with respect to lipid) from vesicles loaded with NaCl and dispersed in Na2SO4 (see Fig. 4 and ESI†). g Evidence for sulfate transport (no or signal) using 33S NMR techniques and Mn2+ as paramagnetic agent (see Fig. 7 and ESI†). h Evidence for sulfate transport (no or signal) using 33S NMR techniques and Fe3+ as paramagnetic agent (see Fig. 8 and ESI†). i C log P values calculated using Spartan '08 for Macintosh (Ghose–Crippen model) (values taken from ref. 15). j EC50,270s values taken from ref. 15. k Result from 13C NMR experiment taken from ref. 14. l n.m. = not measured. m C log P values calculated using Spartan '10 for Macintosh (Ghose–Crippen model). | ||||||||||
| 1 | 2.06i | 5.60j | Partialk | n.m.l | +0.15 | No | 8.8 | No | No | Cl−/NO3− antiport and minimal Cl−/HCO3− antiport |
| 2 | 5.50i | 0.31j | Yesk | n.m.l | +0.15 | No | 33.4 | No | No | Cl−/NO3− and Cl−/HCO3− antiport |
| 3 | 4.43i | 0.24j | Yes | Partial | +0.00 | No | 84.3 | Signal | Signal | Cl−/NO3−, Cl−/HCO3−, Cl−/SO42− and HCO3−/SO42− antiport |
| 4 | 7.87i | 0.032j | Yes | Partial | −1.00 | No | 96.4 | Signal | Signal | Cl−/NO3−, Cl−/HCO3−, Cl−/SO42− and HCO3−/SO42− antiport (sulfate probably transported as HSO4−) |
| 5 | 7.59i | 0.0044j | Yes | Partial | −0.80 | No | 63.3 | Signal | Signal | Cl−/NO3−, Cl−/HCO3−, Cl−/SO42− and HCO3−/SO42− antiport (sulfate probably transported as HSO4−) |
| 6 | 11.03i | 0.042j | Partial | Partial | +0.15 | No | 16.0 | Signal | Signal | Cl−/NO3− antiport and small amounts of Cl−/HCO3−, Cl−/SO42− and HCO3−/SO42− antiport |
| 7 | 6.97m | n.m.l | No | No | n.m.l | No | 4.7 | No | No | No transport |
| 8 | −3.62m | 3.11 | No | No | +0.10 | No | 16.4 | No | No | Cl−/NO3− antiport |
| 9 | 0.71m | 1.13 | Yes | Partial | +0.00 | No | 80.2 | No | Signal | Cl−/NO3−, Cl−/HCO3−, Cl−/SO42− and HCO3−/SO42− antiport and Mn2+/SO42− symport |
In contradistinction to these results, even though the EC50,270s values for 8–9 are much higher than for tren compounds 1–6, compound 9 is still one of the best sulfate transporters in the series. This implies that compound 9 is more selective for transporting sulfate than tren-based compounds 3–5 that still preferentially transport the more lipophilic chloride and nitrate anions. The reason for this increased selectivity for sulfate transport is probably due to the increased selectivity for sulfate binding, due to the cage-like structure of 9 that is ideal for tetrahedral anions such as sulfate that can be bound inside the cavity via nine hydrogen bonds.17–19 The difference in transport ability between the various cyclopeptides 7, 8 and 9 is most likely a combination between lipophilicity, anion affinity and size of the cage. Compound 7 is the most lipophilic receptor, but the large open cage results in reduced anion affinity and a reduced ability to screen the anion from the lipid environment and therefore it is unable to transport anions. The anion transport ability of cyclopeptide 8 on the other hand might be hindered by the highly polar character of this receptor (and hence reduced membrane partitioning) and by the small cage size in which anions can be too tightly bound and cannot be released quickly once the transporter crosses the membrane. Finally, compound 9 shows the correct balance between lipophilicity, high anion affinity and a cage that is small enough to screen the anion from the membrane, but large enough to allow fast in-and-out movement of the anion during transport. Furthermore, compound 9 is the only receptor that can also function via a Mn2+/SO42− symport mechanism, a process that can be aided by the overall neutral complex of this ion-pair and the many metal-coordinating N and O atoms in the cyclopeptide ring. It is still possible that the activity of 9 is due to the formation of a channel or pore that is small enough to keep calcein inside but large enough to cause leakage of small ions, but the Hill coefficient of 1.1 and the difference in bicarbonate transport ability in the presence or absence of chloride (Fig. 3) and the difference in sulfate transport in the presence of Mn2+ or Fe3+ are evidence that lead us to suggest otherwise. Even though all tris-ureas and tris-thioureas 1–9 still transport chloride, nitrate and bicarbonate better than sulfate (with varying degrees of selectivity), it is a first step in developing sulfate selective anion carriers that could be used in the treatment of sulfate transport related diseases and it shows that both lipophilicity and sulfate affinity/selectivity are of the utmost importance in the transport of the highly hydrophilic sulfate anion.
Conclusions
In conclusion, we have developed a novel method using 33S NMR that can be employed to directly monitor sulfate transport in vesicles. By taking advantage of the paramagnetic properties of Mn2+ or Fe3+ it is possible to determine whether [33S]sulfate is located inside or outside the vesicle and hence to establish sulfate transport. We have used this technique to show for the first time that trans-membrane sulfate transport may be mediated by small molecules based on tren and cyclic peptide scaffolds. We believe this work provides a good starting point for the development of new sulfate transporters and provides an unambiguous method to characterise their transport properties in vesicles.Acknowledgements
The authors thank the European Cooperation in Science and Technology (COST) action CM1005 “Supramolecular Chemistry in Water” and EU FP7 project East-NMR (grant no. 228461) for funding. PAG thanks the University of Southampton and A*STAR for a post-graduate scholarship (NB) and the EPSRC for post-doctoral fellowships (MW & CJEH) (EP/J009687/1 and EP/G002576/1) and a studentship (LEK). PAG also thanks the Royal Society and the Wolfson Foundation for a Royal Society Wolfson Research Merit Award. DM and JP thank the Slovenian Research Agency (ARRS, P1-0242 and J1-4020). KAJ thanks the Australian Research Council (DP0877726) and the University of Sydney International Program Development Fund for financial support. The authors would also like to thank Alistair J. Clark from the University of Southampton for his help with SEM-EDX.Notes and references
- Advances in Molecular and Cell Biology, ed. E. E. Bittar and P. Michael, Elsevier, 2006, vol. 38 CAS; T. J. Jentsch, V. Stein, F. Weinreich and A. A. Zdebik, Physiol. Rev., 2002, 82, 503 CAS; F. M. Ashcroft, Ion Channels and Disease, Academic Press, San Diego and London, 2000 Search PubMed.
- For recent reviews: J. T. Davis, O. Okunola and R. Quesada, Chem. Soc. Rev., 2010, 39, 3843 RSC; P. R. Brotherhood and A. P. Davis, Chem. Soc. Rev., 2010, 39, 3633 RSC; P. A. Gale, Acc. Chem. Res., 2011, 44, 216 CrossRef CAS PubMed; S. Matile, A. Vargas Jentzsch, J. Montenegro and A. Fin, Chem. Soc. Rev., 2011, 40, 2453 RSC.
- C. Higgins, Nature, 1992, 358, 536 CrossRef CAS PubMed; J. Y. Choi, D. Muallem, K. Kiselyov, M. G. Lee, P. J. Thomas and S. Muallem, Nature, 2001, 410, 94 CrossRef PubMed.
- For recent reviews: N. Busschaert and P. A. Gale, Angew. Chem., Int. Ed., 2013, 52, 1374 CrossRef CAS PubMed; P. A. Gale, R. Pérez-Tomás and R. Quesada, Acc. Chem. Res., 2013, 46, 2801–2813 CrossRef PubMed.
- I. Alfonso and R. Quesada, Chem. Sci., 2013, 4, 3009 RSC; S. J. Moore, C. J. E. Haynes, J. Gonzalez, J. L. Sutton, S. J. Brooks, M. E. Light, J. Herniman, G. J. Langley, V. Soto-Cerrato, R. Pérez-Tomás, I. Marques, P. J. Costa, V. Félix and P. A. Gale, Chem. Sci., 2013, 4, 103 RSC; S. J. Moore, M. Wenzel, M. E. Light, R. Morley, S. J. Bradberry, P. Gómez-Iglesias, V. Soto-Cerrato, R. Pérez-Tomás and P. A. Gale, Chem. Sci., 2012, 3, 2501 RSC; S. Matsuyama, J. Llopis, Q. L. Deveraux, R. Y. Tsien and J. C. Reed, Nat. Cell Biol., 2000, 2, 318 CrossRef CAS PubMed; J. L. Sessler, L. R. Eller, W.-S. Cho, S. Nicolaou, A. Aguilar, J. T. Lee, V. M. Lynch and D. J. Magda, Angew. Chem., Int. Ed., 2005, 44, 5989 CrossRef PubMed; T. Sato, H. Konno, Y. Tanaka, T. Kataoka, K. Nagai, H. H. Wasserman and S. Ohkuma, J. Biol. Chem., 1998, 273, 21455 CrossRef PubMed; S. Ohkuma, T. Sato, M. Okamoto, H. Matsuya, K. Arai, T. Kataoka, K. Nagai and H. H. Wasserman, Biochem. J., 1998, 334, 731 Search PubMed.
- Y. Marcus, J. Chem. Soc., Faraday Trans., 1991, 87, 2995 RSC.
- S. K. Berezin and J. T. Davis, J. Am. Chem. Soc., 2009, 131, 2458 CrossRef CAS PubMed; B. A. McNally, A. V. Koulov, T. N. Lambert, B. D. Smith, J.-B. Joos, A. L. Sisson, J. P. Clare, V. Sgarlata, L. W. Judd, G. Magro and A. P. Davis, Chem.–Eur. J., 2008, 14, 9599 CrossRef PubMed.
- P. Talukdar, G. Bollot, J. Mareda, N. Sakai and S. Matile, J. Am. Chem. Soc., 2005, 127, 6528 CrossRef CAS PubMed.
- I. Ravikumar and P. Ghosh, Chem. Soc. Rev., 2012, 41, 3077 RSC; L. R. Eller, M. Stȩpień, C. J. Fowler, J. T. Lee, J. L. Sessler and B. A. Moyer, J. Am. Chem. Soc., 2007, 129, 11020 CrossRef CAS PubMed; R. Custelcean, P. Remy, P. V. Bonnesen, D.-e. Jiang and B. A. Moyer, Angew. Chem., Int. Ed., 2008, 47, 1866 CrossRef PubMed; R. Custelcean, A. Bock and B. A. Moyer, J. Am. Chem. Soc., 2010, 132, 7177 CrossRef PubMed; B. Akhuli, I. Ravikumar and P. Ghosh, Chem. Sci., 2012, 3, 1522 RSC.
- G. Lumetta, in Fundamentals and Applications of Anion Separations, ed. B. Moyer and R. Singh, Springer, USA, 2004, pp. 107–114 CrossRef CAS PubMed; B. A. Moyer, R. Custelcean, B. P. Hay, J. L. Sessler, K. Bowman-James, V. W. Day and S.-O. Kang, Inorg. Chem., 2013, 52, 3473 CrossRef CAS PubMed.
- For examples: S. O. Kang, V. W. Day and K. Bowman-James, Org. Lett., 2009, 11, 3654 CrossRef CAS PubMed; P. A. Gale, J. R. Hiscock, C. Z. Jie, M. B. Hursthouse and M. E. Light, Chem. Sci., 2010, 1, 215 RSC; J.-i. Kim, H. Juwarker, X. Liu, M. S. Lah and K.-S. Jeong, Chem. Commun., 2010, 46, 764 RSC.
- D. Markovich, Physiol. Rev., 2001, 81, 1499 CAS.
- J. Hästbacka, A. de la Chapelle, M. M. Mahtani, G. Clines, M. P. Reeve-Daly, M. Daly, B. A. Hamilton, K. Kusumi, B. Trivedi, A. Weaver, A. Coloma, M. Lovett, A. Buckler, I. Kaitila and E. S. Lander, Cell, 1994, 78, 1073 CrossRef CAS; A. Superti-Furga, A. Rossi, B. Steinmann and R. Gitzelmann, Am. J. Med. Genet., 1996, 63, 144 CrossRef.
- N. Busschaert, P. A. Gale, C. J. E. Haynes, M. E. Light, S. J. Moore, C. C. Tong, J. T. Davis and W. A. Harrell, Jr, Chem. Commun., 2010, 46, 6252 RSC.
- N. Busschaert, M. Wenzel, M. E. Light, P. Iglesias-Hernández, R. Pérez-Tomás and P. A. Gale, J. Am. Chem. Soc., 2011, 133, 14136 CrossRef CAS PubMed.
- I. Martí, J. Rubio, M. Bolte, M. I. Burguete, C. Vicent, R. Quesada, I. Alfonso and S. V. Luis, Chem.–Eur. J., 2012, 18, 16728 CrossRef PubMed.
- P. G. Young and K. A. Jolliffe, Org. Biomol. Chem., 2012, 10, 2664 CAS.
- P. G. Young, J. K. Clegg, M. Bhadbhade and K. A. Jolliffe, Chem. Commun., 2011, 47, 463 RSC.
- P. G. Young, J. K. Clegg and K. A. Jolliffe, Supramol. Chem., 2011, 24, 77 CrossRef.
- B. D. Smith and T. N. Lambert, Chem. Commun., 2003, 2261 RSC.
- A. V. Koulov, T. N. Lambert, R. Shukla, M. Jain, J. M. Boon, B. D. Smith, H. Li, D. N. Sheppard, J.-B. Joos, J. P. Clare and A. P. Davis, Angew. Chem., Int. Ed., 2003, 42, 4931 CrossRef CAS PubMed.
- A. V. Hill, Biochem. J., 1913, 7, 471 CAS.
- T. P. W. McMullen, R. N. A. H. Lewis and R. N. McElhaney, Curr. Opin. Colloid Interface Sci., 2004, 8, 459 CrossRef CAS PubMed; J. C. M. Holthuis, G. van Meer and K. Huitema, Mol. Membr. Biol., 2003, 20, 231 Search PubMed; W. F. D. Bennett, J. L. MacCallum and D. P. Tieleman, J. Am. Chem. Soc., 2009, 131, 1972 CrossRef PubMed.
- S. Bhosale and S. Matile, Chirality, 2006, 18, 849 CrossRef CAS PubMed.
- J. T. Davis, P. A. Gale, O. A. Okunola, P. Prados, J. C. Iglesias-Sánchez, T. Torroba and R. Quesada, Nat. Chem., 2009, 1, 138 CrossRef CAS PubMed.
- R. R. C. New, in Liposomes: a Practical Approach; IRL Press, Oxford, 1990, pp. 131–134 CrossRef CAS PubMed; V. Sidorov, F. W. Kotch, G. Abdrakhmanova, R. Mizani, J. C. Fettinger and J. T. Davis, J. Am. Chem. Soc., 2002, 124, 2267 CrossRef CAS PubMed; T. N. Lambert, J. Middleton Boon, B. D. Smith, M. Nieves Perez-Payan and A. P. Davis, J. Am. Chem. Soc., 2002, 124, 5276 CrossRef PubMed; S. Licen, C. Coppola, J. D'Onofrio, D. Montesarchio and P. Tecilla, Org. Biomol. Chem., 2009, 7, 1060 Search PubMed.
- K. Kano and J. H. Fendler, Biochim. Biophys. Acta, 1978, 509, 289 Search PubMed; N. R. Clement and J. M. Gould, Biochemistry, 1981, 20, 1534 CrossRef CAS.
- J. Biwersi, B. Tulk and A. S. Verkman, Anal. Biochem., 1994, 219, 139 CrossRef CAS PubMed.
- E. G. Schneider, J. C. Durham and B. Sacktor, J. Biol. Chem., 1984, 259, 14591 Search PubMed; J. B. Pritchard and J. L. Renfro, Proc. Natl. Acad. Sci. U. S. A., 1983, 80, 2603 CrossRef CAS; H. Lohi, M. Kujala, S. Mäkelä, E. Lehtonen, M. Kestilä, U. Saarialho-Kere, D. Markovich and J. Kere, J. Biol. Chem., 2002, 277, 14246 CrossRef PubMed.
- C. J. E. Haynes, S. J. Moore, J. R. Hiscock, I. Marques, P. J. Costa, V. Félix and P. A. Gale, Chem. Sci., 2012, 3, 1436 RSC; V. Saggiomo, S. Otto, I. Marques, V. Felix, T. Torroba and R. Quesada, Chem. Commun., 2012, 48, 5274 RSC; N. Busschaert, S. J. Bradberry, M. Wenzel, C. J. E. Haynes, J. R. Hiscock, I. L. Kirby, L. E. Karagiannidis, S. J. Moore, N. J. Wells, J. Herniman, G. J. Langley, P. N. Horton, M. E. Light, I. Marques, P. J. Costa, V. Felix, J. G. Frey and P. A. Gale, Chem. Sci., 2013, 4, 3036 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures, Hill plots, cholesterol test, 13C NMR tests, symport tests, HPTS pH change tests, calcein leakage assay, lucigenin tests, 33S NMR tests on all compounds with Mn2+ and Fe3+, SEM-EDX experiments. See DOI: 10.1039/c3sc52006d |
| ‡ Present address: Fraunhofer IKTS, 01217, Dresden, Germany. |
| This journal is © The Royal Society of Chemistry 2014 |