The impact of chemistry education research on practice: a cautionary tale
Keith S.
Taber
Faculty of Education, University of Cambridge, Cambridge, UK. E-mail: kst24@cam.ac.uk
Chemistry education research (CER) however has not traditionally enjoyed support for research-for-its-own-sake enquiries. In part this reflects the kinds of academic area education is – an inherently applied field of work. Arguably chemistry education is more akin to chemical engineering than pure chemistry in this sense – where researchers are expected to work on problems that have been identified as having clear relevance to practical concerns. As educational research generally has potential to inconvenience teachers and learners, and indeed even disrupt normal educational activity, there is often also a strong ethical basis for not undertaking research on something ‘just to see what would happen if’ for no better reason than our natural curiosity (see the editorial in CERP15(2) (Taber, 2014)). Rather programmes of research are expected to respond to well recognised issues and challenges in teaching and learning. So we should expect research to have effects on practice.
The highs and lows of being a researcher in chemistry education
I have recently had good reason to ponder the issue of research impact in CER. On the positive side, I was extremely honoured to have been named as the Royal Society of Chemistry's (RSC) Education Award winner 2014. Scientific societies have a long tradition of giving awards and prizes in different subfields, and the inclusion of education as an area of scholarly activity deserving attention in this way is one recognition of the importance of this area of work to chemistry. The award was made for “extensive research that has contributed significantly to the teaching and learning of chemistry concepts”. This is extremely rewarding at a personal level, especially as editing CERP means that I am well placed to recognise just how many other colleagues have made important contributions to chemistry education through their research. However, as I recount below, I have also had reason recently to reflect on how difficult it is for research to influence teaching when its recommendations are contrary to well-established educational customs and practice.The national educational research association in the UK, responding to the inclusion of evidence of research impact in national evaluations of research undertaken in the universities refers to “how education impacts on everyone and how a strong research discipline can ensure that individual lives and communities are transformed through education” (British Educational Research Association, 2013, p. 4). This raises the questions of both how we know research has impact at the level of teaching and learning, and indeed how this happens, given that:
What we know for sure is that educational research generally does not have an immediate impact on policy or practice; indeed it may take many years for the insights from research to filter through. Unlike some areas of research that have the potential to impact on society, it cannot be represented or distilled into simple one line actions. It needs to be interpreted and mediated in a variety of processes to accommodate different circumstances (Gardner, 2011, p. 559).
I am extremely privileged to have been supported by the RSC to undertake work that was directly designed to help teachers access and apply research – through one of a number of annual teacher fellowships that were awarded for projects considered capable of supporting the teaching of chemistry at school or college level. (In the UK references to college level usually mean students in pre-university courses.) My project concerned the development of classroom materials to help teachers identify and so challenge students' alternative conceptions (‘misconceptions’) in core areas of chemistry. The RSC not only supported this project by funding my release from all other academic responsibilities for a full academic year (during which time the project was hosted by the University of London Institute of Education) but though a commitment to widespread dissemination of the outcomes. Two A4 sized books on ‘Chemical Misconceptions’ (Taber, 2002a, 2002b) were published and sent at the RSC's expense to secondary schools, further education colleges and university teacher education departments nationally. Moreover, the classroom materials themselves have been made available free from the RSC's website as electronic downloads both as pdf files and as editable word-processing files.
Of course, making resource-informed materials available does not necessarily lead to uptake. Teachers may be too busy to properly evaluate materials and consider how they might integrate them into teaching. Moreover, research-informed resources only lead to research-informed practice when they are used in ways consistent with the original intention. Too often such materials may be adopted without the perspective from within which they were designed – especially when there is no ongoing professional development support. These particular materials were designed to support teachers in applying an approach to teaching (one book provided the background, drawing extensively upon ideas and examples from chemistry teaching and learning; the other book provided a set of classroom activities, that were each accompanied by teacher guidance) but it is known that teachers can be very good at dissecting activities from their intended pedagogic contexts and then fitting them into existing ways of teaching. For example, the diagnostic materials were not intended – nor in general were they especially suitable for – use in summative assessment, but there was nothing to stop teachers using them as end-of-topic tests.
With many projects the researcher (or resource developer) does their work, distributes it (or often simply makes it available and hopes the target audience find it), and then trusts it may be taken up – and will have an effect. Anecdotal evidence from chance meetings and the like may reinforce an impression that there has been impact, but unless the work is linked with or adopted into some major national initiative it may be difficult to trace any effect.
The RSC, who have over the years invested significantly in supporting chemistry education in schools and colleges through resource development and various other initiatives, commissioned an independent evaluation of a selection of their educational resources from a team based in the UK's Open University.† The evaluation (Murphy et al., 2004) provided evidence that teachers had accessed the ‘Chemical Misconceptions’ materials, were using the activities, and that they were contributing to student learning. The report suggested that engaging with the materials had changed teacher thinking about student learning, and had an on-going effect on teachers' classroom practice, and that the materials were used by experienced teachers in their mentoring of new chemistry teachers. The evaluators commented that the classroom materials reflected a successful embedded pedagogic strategy. It is very helpful (and reassuring) to be provided with evaluation of this kind, yet in my experience it is very unusual for educational researchers to be provided with such clear feedback on the impact of their work. Moreover, this was a project that was primarily about the transfer of research findings into the classroom, drawing on research that had already been undertaken (including, but by no means restricted to, my own research).
I have little doubt that the positive impact of this project reflects the commitment of the RSC as a learned society to invest and engage in supporting school and college teaching in chemistry, and that this provided a relatively rare opportunity to develop research-informed materials that would actually be widely disseminated so that teachers had ready access to them. Much educational research does not benefit from such levels of support in interpretation and dissemination. (The cost of printing books and distributing them to thousands of schools by post is clearly considerable.)
Moreover, despite the positive findings of the evaluation report, I am under no illusions that even such a well-supported initiative can immediately solve longstanding and insidious problems in teaching and learning chemistry. I was reminded of this very recently when I came across a display of the latest batch of chemistry textbooks at a teacher's conference. Flicking through the first book I picked up I came across a figure labelled as ‘ionic bonding’. This was the familiar image of a sodium atom adjacent to a chlorine atom, with an electron being transferred from the sodium atom to the chlorine to atom (of the general form shown in Fig. 1).
The figure was intended to represent ionic bonding, and I suspect most readers will have seen similar figures ‘of’ ionic bonding in school books. Yet this image does not effectively represent ionic bonding. Ionic bonding is about electrostatic forces binding a lattice of ions, not about electron transfer. The diagram shows ion formation, which is clearly something quite different. Certainly it is necessary for ions to exist to have ionic bonding, so ions must be formed for this to happen. But by the same logic, suitable glassware has to be constructed before we can carry out a distillation: but school texts books do not tend to present images of glassblowing labelled as ‘distillation’, and it would seem quite bizarre if they did.
Perhaps textbook authors think that representing ionic bonding in terms of lattice interactions is too complex and abstract for students who are still novices at chemistry, and consider ‘ionic-bonding-as-electron-transfer’ images (such as Fig. 1) as a kind of pedagogic simplification – a teaching model. This might be a potential argument if Fig. 1 can be understood as a useful simplification of ionic bonding (after all, returning to my earlier argument, no one would suggest that the manufacturing of a reflux condenser offers a simplified teaching model of distillation).
Whether the idea of an electron being transferred between atoms is actually inherently any easier to understand than the clumping together of cations and anions is a moot point (as if not, the simplification argument falls down in its own terms). What I am clear about, because my own research demonstrated this, is that advanced students asked to learn about ionic bonding in terms of a lattice of charged ions found that having previously acquired the idea that ionic bonding was electron transfer interfered with the required learning. The teaching model (if we are generous, and for the moment grant it that status) acted as a learning impediment. The electron transfer model supported a ‘molecular’ conception of materials such as common salt – so students tended to think that there were NaCl units (molecules, or molecule like units – pseudo-molecules) within sodium chlorine that were held together by strong ionic bonds (the result of the hypothetical electron transfer) and which were only linked to other NaCl units in the lattice by weak interactions that were not really proper bonds. Such a mental model of NaCl does not explain properties such as a high melting point (as the weak interactions between the pseudo-molecules should be readily disrupted) or conduction when molten (as the pseudo-molecules have no net charge), and leads students to expect the solvated species in solution to be NaCl ‘molecules’.
The magic of chemistry
Indeed, some students find it quite difficult to shift to thinking about bonding as a physical process, due to electrical forces, at all, when they have been introduced to bonding through non-physical models such as that shown in Fig. 1. Quite often introductory chemistry presents ionic bonding in terms of electron transfer, and covalent bonding in terms of an equally non-physical notion of ‘electron sharing’. As chemists we tend to appreciate that the sharing notion is metaphorical, but if that is the only ‘explanation’ students are offered when they first meet the concept of chemical bonding then they have to find ways to make sense of what bonding is about. One problem with these ‘teaching models’ is that they avoid discussing any physically viable mechanism for processes, leaving the impression that something magical is going on: in particular, a need or desire for ‘full electron shells’ drives atoms to bond.Atomic nirvana
Given an explanatory vacuum for understanding chemical bonding students readily adopt notions of the stability of full shells, or octets of electrons, as being the basis of a driving force in chemistry. Species with these configurations are assumed to be stable (even when highly charged, e.g. C4−) and atoms are imbued with a kind of sentience that allows them to both know whether they have such a configuration, and – if not – to do something about it.From this perspective NaCl forms an ionic bond because the sodium atom wants to loose an electron (to leave it with a full electron shell) and the chlorine wants want to gain one (to acquire a full outer shell). Of course, the process shown in Fig. 1 does not even actually allow the chlorine atom to obtain a full outer shell, as that would require a further ten electrons to give Cl11− (a highly charged non-viable species that many students judge more stable than the neutral atom).
I expect this narrative is familiar to most readers, many of whom may have actually been taught in this way, or at least seen school text book accounts that present (or leave readers to infer) this narrative. The only obvious strength of this ‘teaching model’ is that students seem to readily understand, accept and learn the idea that atoms strive for some kind of atomic nirvana through obtaining full shells – so it provide students with a mental model of bonding (and, for example, how and why reactions occur) that allows them to think about chemistry at the submicroscopic scale and feel they understand what is going on.
Against this strength, however, there are some serious weaknesses. The most important being that the account is not true to the science, and becomes a major impediment to learning more advanced models that are based on physical explanations to do with charges and forces. As pointed out above, this alternative conceptual framework (i.e. set of linked conceptions) leads to the wrong predictions of properties in ionic materials. It also leads to misjudgments about the relative stability of many ions and atoms, as those with full outer shells or octets are assumed to be more stable whereas usually a neutral species is actually more stable (in the absence of a stabilising environment such as occurs during solvation). It also leads to nonsensical arguments about why reactions occur: such as students arguing that hydrogen and fluorine react because the atoms want to achieve full shells…in response to a question that gives an equation for the reaction showing the reagents are molecular (so already have ‘full outer shells’).
In the beginning
Perhaps the reader who feels I am being a bit harsh might cling to the point about Fig. 1 representing ion formation, which is necessary for ionic bonding. However, I would argue even this is a weak argument for using representations such as Fig. 1 when teaching about ionic bonding. Of course, ionic bonding relies upon the presence of ions, and therefore at some point those ions must have been formed. However (1) I am not convinced that the ions need to have been formed by electron transfer between atoms; and (2) if they were, it still was almost certainly not how the ionic bonding in any particular salt sample came about.The scientific model of the process whereby elements are formed in stars (which in effect means the nuclei of different heavier elements are formed, as the temperatures are much too great for neutral atoms to exist) and distributed through supernovae does not have a discrete clean stage when all the material is atomic. The interstellar medium contains atoms, but also molecules and ions. Although there seems to be a psychological preference for beginning chemical narratives with discrete atoms the universe is not minded to organise matter in atomic form before it can react. In reality the elements are formed as plasma and do not all then become discrete neutral atoms on their way to forming compounds.
Certainly – coming back to earth – when we think of how a sample of sodium chloride might be formed in the school laboratory, Fig. 1 has little relevance. A teacher may demonstrate binary synthesis between sodium (a metal, with a metallic lattice, not discrete atoms) and chlorine (a molecular gas, not discrete atoms). More likely students will form sodium chloride by neutralisation and evaporation. They will produce sodium chloride with ionic bonding between a lattice of cations and anions. The reaction does not involve any process that could be reasonably represented by Fig. 1. Sodium ions that are already present in sodium hydroxide, and become solvated in solution, then bind with chloride ions that were (already present and) solvated in the acid solution. No ion formation occurs. No electron transfer is needed. Fig. 1 has no relevance.
Fig. 1 does not even represent an energetically viable process. Whilst the electron affinity of chlorine means that energy is released on the formation of the chloride ion, this is insufficient to match the ionisation energy needed to remove the electron from the sodium atom.
None of this should be news. In terms of my own research with English students, I reported on these problems many years ago. The ways that English college students understood chemical bonding and talked about chemical processes in anthropomorphic terms were the subject of papers in research journals (e.g., Taber and Watts, 1996; Taber, 1998) and the particular issues about understanding ionic bonding were highlighted in periodicals read widely by teachers (Taber, 1994, 1997). Moreover, these particular issues (among others) were highlighted in the RSC project and the classroom resources discussed above: materials that were judged to have been influential on teaching and learning (Murphy et al., 2004). Yet textbook authors continue to re-use representations of the kind shown in Fig. 1, despite these being both bad science, and bad pedagogy. Text books being produced today are still presenting a notion of ionic bonding that has been pointed out to be technically incorrect, internally inconsistent, dependent for causality on the actions of sentient atoms, and that has been found to act as an impediment to the learning of scientifically appropriate models.
These problems are certainly not unique in the English context. Research from Australia had previously identified some of the same issues with students' understanding of ionic bonding (Butts and Smith, 1987), and the general patterns of alternative conceptions identified among English college students have since been found to have resonances with students in a range of other national contexts (Taber, 2013). In the English context, however, textbook authors who perpetuate the ‘electron transfer’ notion of ionic bonding have a powerful ally: the government's Department of Education. A previous government initiative to strengthen teaching had recommended the RSC materials on Chemical Misconceptions to schools (Key Stage 3 National Strategy, 2003), but despite this, the government ministry recently published a new draft curriculum document (Department for Education, 2014) that sets out as canonical knowledge for 16 year old students in England that: “atoms bond either by transferring electrons from one atom to another or by sharing electrons” (p. 11).
As long as governments set out such dubious statements as target knowledge for learners it is hardly surprising that textbook authors will choose to maintain flawed teaching models rather than look to offer a scientifically acceptable model of the chemistry. Clearly in this case the impact of widely disseminated research has not been sufficient to influence the government ministry which decides what students should be taught.
Research informing practice (but not always quickly and directly)
That is certainly not to say that I am downbeat about the influence of research on educational practice. I do feel that in my time working in science education (somewhat over thirty years now) I have seen research-informed ideas gradually taken up as part of educational discourse, and practices shift in response to recommendations from research. General constructivist principles about the way learners build up their understanding of topics and the importance of their prior knowledge have become a basis of widespread pedagogy – even if sometimes at the cost of being somewhat trivialised in the process (Taber, 2010). Arguments about the importance of dialogue to effective classroom learning (Mortimer and Scott, 2003; Mercer et al., 2004) are becoming widely adopted. There are many examples of specific innovations in teaching, resources, assessment that are disseminated through the CER community (as the articles in CERP clearly demonstrate). In terms of classroom practice, the current ripple of interest in the flipped classroom (Smith, 2013) can be seen as an evolution of the idea of prelabs to prepare students to learn effectively from practical work (Johnstone et al., 1994). Diagnostic instruments informed by research into students' ideas (Treagust, 1988) are being applied to inform teaching interventions (Regan et al., 2011). There are many other examples.The problem with looking for research impact, is that it is unreasonable to expect most individual studies to directly bring about widespread changes in practice that can be traced back to that particular research (see Fig. 2). For one thing, most research studies are parts of more extensive research programmes where understanding, and recommendations for practice, develop iteratively over time. Each discrete study adds a little to understanding within the research community. In addition, practitioners often borrow or copy good ideas from each other (deliberately or sometimes without even realising) and even if the teacher whose practice is taken as a model knows what research has influenced that practice, this information is seldom made explicit as good practice is spread among a community of practice. In any case, often practitioners do not know the details of the research that informs their own practice. Some teachers read research journals such as CERP, but this is not the norm at school levels – and many teachers at university level who are not themselves active in pedagogic inquiry are too busy keeping up with their own research fields to regularly read reports of educational research.
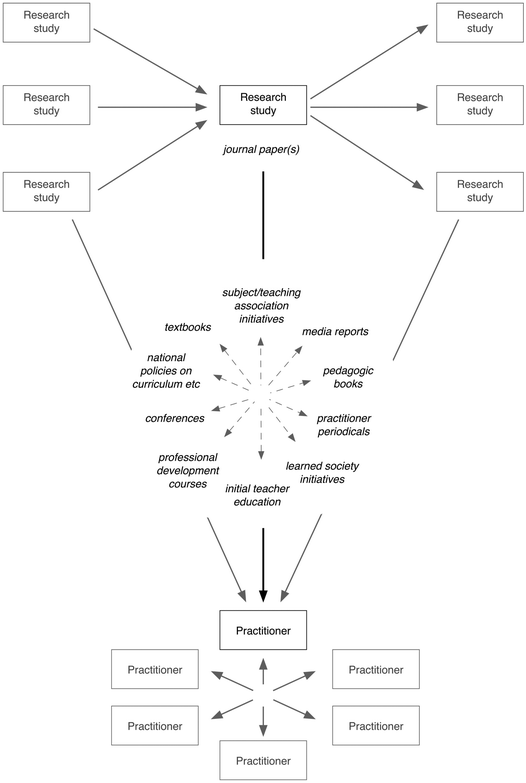 | ||
| Fig. 2 The influence of research on educational practice is seldom in the form of clearly identifiable direct impact. | ||
Research results and recommendations may be reported in other publications such as practitioner journals or even sometimes mainstream media – but the link to the original research papers may not always be explicit. (Often practitioner journals look for articles that cite short bibliographies of accessible further reading, rather than academic citations of the kind expected in research journals.) Teaching associations or networks, and learned societies and professional bodies, may sometimes produce digests of research findings or research-based initiatives. Research will often be read by those responsible for teacher education and professional development – but their priority is often to translate bodies of research into core recommendations for practice that teachers (especially those setting out as new teachers) can readily engage with.
Of course these various potential intermediaries between research reports and practitioner knowledge interact in complex ways. Any particular research study will have been read by a finite number of people, whose thinking will have been influenced in different ways and to different extents, and any resulting shifts in thinking may influence their own work (in their own research, preparing teachers, in informing educational policies of learned societies etc.) in different ways and to different degrees – and they then engage in relevant discourse with others undertaking similar or different work (e.g. the textbook author who reads a policy document from a teaching association written by an association officer or consultant informed by various research). Any particular teacher will be informed by any reading of research, plus any reading of secondary literature, their own pedagogic preparation (which may be quite limited if they are teaching in higher education contexts), the practice of those about them, and so forth. There is a complex network of individuals in different roles each influenced by and influencing many others. Much of the time most of those individuals are only very vaguely aware of most of the research studies that have influenced the ideas, language and practice that is part of the milieu in which they work.
A physical analogy for the impact of educational research
Ultimately any simple notion of measuring some kind of ‘cause and effect’ between educational research and educational practice is highly problematic. The process by which research influences practice reminds me of the process by which nuclear reactions in the sun lead to the solar radiation incident on the earth. The photons arriving at earth today as infrared and visible radiation are certainly a result of the nuclear furnace in the sun's core. However those photons were not directly produced in the sun's core. Rather the radiation generated by the nuclear processes is absorbed in and supplies energy to the surrounding ‘radiation zone’ – leading to the emission of further (eventually less energetic) radiation that is then re-absorbed, and so on. This process occurs a great many times before a photon from nearer the sun's surface eventually radiates into space without further absorption. The structure of the sun as a whole is in part due to the myriad processes of radiation emission and absorption that maintains the conditions (temperature, pressure) in different parts of the system – that in turn determine such features as the mean free path of any particular photon in that region of the sun. Indeed it is suggested that the time delay between the core nuclear process and a (indirectly) resulting photon eventually leaving the sun is at least of the order of tens of thousands of years. It is strictly incorrect then to say that a photon arriving at earth now was the direct result of nuclear interactions in the sun: rather it is a very indirect and slow process. Despite the convoluted chain of events, which both depend upon and maintain the sun as a complex radiative system, it is still clear that indirectly the nuclear reactions are the cause of the solar radiation we all rely on for life to continue on earth.Something similar is true about the impact of education research. The process is convoluted and diffuse, and very difficult to track, and indeed sometimes there is a considerable delay before research impacts on practice (although, thankfully, not quite as long as it takes radiation to emerge from the sun). Despite this, teaching chemistry is improving as a result of chemistry education research. The occasional disappointments and reversals remind us just how complex the process is, and why researchers have a responsibility to do what they can to help disseminate and reconceptualise research in ways that can influence teaching.
References
- British Educational Research Association, (2013), Why educational research matters: a briefing to inform future funding decisions, London: British Educational Research Association.
- Butts B. and Smith R., (1987), HSC Chemistry Students' Understanding of the Structure and Properties of Molecular and Ionic Compounds, Res. Sci. Educ., 17(1), 192–201.
- Department for Education, (2014), Science key stage 4: June 2014 Draft, London: Department for Education.
- Gardner J. (2011), Educational research: what (a) to do about impact!, Brit. Educ. Res. J., 37(4), 543–561, DOI: http://10.1080/01411926.2011.596321.
- Johnstone A. H., Sleet R. J. and Vianna J. F., (1994), An information processing model of learning: Its application to an undergraduate laboratory course in chemistry, Stud. High. Educ., 19(1), 77–87, DOI: http://10.1080/03075079412331382163.
- Key Stage 3 National Strategy, (2003), Strengthening Teaching and Learning of Particles in Key Stage 3 Science: Notes for Participants, No place of publication given: Department for Education and Skills.
- Mercer N., Dawes L., Wegerif R. and Sams C., (2004), Reasoning as a scientist: ways of helping children to use language to learn science, Brit. Educ. Res. J., 30(3), 359–377, DOI: http://10.1080/01411920410001689689.
- Mortimer E. F. and Scott P. H., (2003), Meaning Making in Secondary Science Classrooms, Maidenhead: Open University Press.
- Murphy P., Jones H. and Lunn S. (2004), The evaluation of RSC materials for schools and colleges: a report, London: The Royal Society of Chemistry.
- Regan A., Childs P. and Hayes S. (2011), The use of an intervention programme to improve undergraduatestudents' chemical knowledge and address their misconceptions, Chem. Educ. Res. Pract., 12(2), 219–227, DOI: 10.1039/C1RP90027G.
- Smith J. D., (2013), Student attitudes toward flipping the general chemistry classroom, Chem. Educ. Res. Pract., 14(4), 607–614, DOI: 10.1039/C3RP00083D.
- Taber K. S., (1994), Misunderstanding the Ionic Bond, Educ. Chem., 31(4), 100–103.
- Taber K. S., (1997), Student understanding of ionic bonding: molecular versus electrostatic thinking?, Sch. Sci. Rev., 78(285), 85–95.
- Taber K. S., (1998), An alternative conceptual framework from chemistry education, Int. J. Sci. Educ., 20(5), 597–608.
- Taber K. S., (2002a), Chemical Misconceptions - Prevention, Diagnosis and Cure: Classroom resources, vol. 2, London: Royal Society of Chemistry.
- Taber K. S., (2002b), Chemical Misconceptions - Prevention, Diagnosis and Cure: Theoretical background, vol. 1, London: Royal Society of Chemistry.
- Taber K. S., (2010), Paying lip-service to research?: the adoption of a constructivist perspective to inform science teaching in the English curriculum context, The Curriculum Journal, 21(1), 25–45.
- Taber K. S., (2013), A common core to chemical conceptions: learners' conceptions of chemical stability, change and bonding, in Tsaparlis G., Sevian H. (ed.), Concepts of Matter in Science Education, Dordrecht: Springer, pp. 391–418.
- Taber K. S., (2014), Ethical considerations of chemistry education research involving “human subjects”, Chem. Educ. Res. Pract., 15(2), 109–113, DOI: 10.1039/C4RP90003K.
- Taber K. S. and Watts M., (1996), The secret life of the chemical bond: students' anthropomorphic and animistic references to bonding, Int. J. Sci. Educ., 18(5), 557–568.
- Treagust D. F., (1988), Development and use of diagnostic tests to evaluate students' misconceptions in science, Int. J. Sci. Educ., 10(2), 159–169, DOI: http://10.1080/0950069880100204.
Footnote |
| † The evaluation sampled a selection of RSC resources. As well as the Chemical Misconceptions volumes, the evaluators also considered other hard copy materials (Classic Chemistry Experiments; Classic Chemistry Demonstrations; Ideas and Evidence), multimedia materials on CDROM (Alchemy?), professional development materials (Improving Teaching and Learning Chemistry using ICT; Using Assessment to Improve Learning in Chemistry and Science) and web-based materials (Joint Earth Science Education Initiative). The full evaluation report is available at www.rsc.org/images/2004evaluation_tcm18-12552.pdf. |
| This journal is © The Royal Society of Chemistry 2014 |

