The flipped classroom for teaching organic chemistry in small classes: is it effective?
Jessica M.
Fautch
Department of Physical Sciences, York College of Pennsylvania, USA. E-mail: jfautch@ycp.edu
First published on 15th December 2014
Abstract
The flipped classroom is a pedagogical approach that moves course content from the classroom to homework, and uses class time for engaging activities and instructor-guided problem solving. The course content in a sophomore level Organic Chemistry I course was assigned as homework using video lectures, followed by a short online quiz. In class, students' misconceptions were addressed, the concepts from the video lectures were applied to problems, and students were challenged to think beyond given examples. Students showed increased comprehension of the material and appeared to improve their performance on summative assessments (exams). Students reported feeling more comfortable with the subject of organic chemistry, and became noticeably passionate about the subject. In addition to being an effective tool for teaching Organic Chemistry I at a small college, flipping the organic chemistry classroom may help students take more ownership of their learning.
Introduction
To college science majors, sophomore-level Organic Chemistry I has a reputation as a scary, “weed-out” course, partially because it is so content-laden and fast-paced in order to address a host of learning goals. The challenges of effectively teaching this course stems from the large amount of content needed to serve the eclectic student population enrolled in the course: biology, chemistry, and pre-professional majors. By the time students begin their sophomore year of college, they are likely to be comfortable with the lecture format of their courses, and feel they can learn well by taking notes in class while the instructor lectures to them. However, passive lecture, without any activities, is largely ineffective with respect to comprehension and retention (Halloun, 1985; Seymour and Hewitt, 1997). Introducing the students to the material through interactive lecturing or other active methods (e.g. problem-based learning, peer-instruction, case-studies, debates, etc.) has been shown to be much more effective in helping students learn (Crouch and Mazur, 2001; Prince, 2004; Lasry et al., 2008).One such active teaching method is flipping (or inverting) the classroom. The flipped classroom is one that is primarily student-centered (active), as opposed to instructor-centered (lecture). Flipping the class removes content from the classroom and places it on the student as homework. The means by which the content is delivered outside of class can vary (i.e. tutorials, readings, videos, vodcasts, lecture-captured videos, etc.). The removal of content from the class period allows the instructor to spend more time actively working with the students, and allows the use of a variety of learning tools. The flipped classroom was initially implemented and reported by economists (Lage et al., 2000) aimed at reducing variability in teaching styles across the classroom in order to increase student performance. Because each instructor was able to implement various activities in order to create an inclusive classroom, the method was successful. Various other disciplines, including pharmacy (Pierce and Fox, 2012), statistics (Strayer, 2012), computer science (Foertsch et al., 2002; Davies et al., 2013), and medicine (McLaughlin et al., 2014) have reported success with implementing the flipped class.
In terms of pedagogy, the flipped classroom method continues to garner a lot of attention. In chemistry, this approach was introduced by two high school teachers, Jonathan Bergmann and Aaron Sams (2012), and has become widely spread among disciplines and curriculum levels. There is a small body of work that discusses the merits of using the flipped method in chemistry courses (Bergmann and Sams, 2012; Arnaud, 2013), with the majority of the cases involving general chemistry in high school. Very little published evidence exists about the effectiveness of the flipped classroom in higher education (Smith, 2013; Teo et al., 2014). Fewer yet are examples of implementation of the flip in sophomore-level organic chemistry (Bradley et al., 2002; Christiansen, 2014).
Given the limited number of reports of the effectiveness of the flipped classroom when implemented with college sophomores in Organic Chemistry I, this paper aims to fill some of the informational gap. Reported here are the results of an implementation of the flipped class in Organic Chemistry I, including student perceptions, student performance (grades), and student ownership of their own learning.
Flipped course design
When considering the option to flip the organic chemistry curriculum, several factors were at play. The biggest issue with the original non-flipped lecture course was that there did not seem to be enough time in the class period for both covering content and getting sufficient practice on problems (i.e. naming, mechanisms, and eventually syntheses). Because the course was not taught solely by one instructor, and a common set of learning objectives was used, the option to remove or revise content was not feasible. In order to create more time during class to help guide students through practice problems, the content needed to be communicated in a different way. Therefore, flipping the class by moving lecture material to videos as homework seemed like a viable option to explore.Course format
The sophomore organic chemistry curriculum at York College (a small college in Pennsylvania, United States) consists of a two-course sequence: Organic I and Organic II. Both courses are structured such that enrollment is capped at 24 students per lecture with separate lab sections of 16 students. The lectures meet 2 days a week for 75 minute sessions. Recitation or discussion sections are not part of either course, and teaching assistants are only present in the laboratory portion of the courses. Students enrolled in the courses represent several majors within the biological and physical sciences.Although the curriculum follows a two-course sequence, only the first course, Organic Chemistry I, was followed for this study and will be commonly referred to as “the course” herein. Additionally, Organic I is offered every semester at the college, and information from each semester was collected, beginning in Fall 2011. In the Fall 2011 semester, a standard sophomore-level Organic Chemistry I lecture was taught as primarily lecture-based (i.e. “non-flipped”), with some active learning (i.e. group problem-solving and reporting) during class. In the Spring 2012 semester the same course was offered again as non-flipped, but the student population was slightly different. The spring course comprises those students who are off-sequence, or out of order. Generally speaking, the students in the spring semesters are not as strong, collectively, as the students in the Fall semesters. In Fall 2012, and every semester thereafter, the Organic I course was taught using the flipped classroom approach. One important note is that Organic I in the Fall 2013 semester was facilitated by a different instructor while the regular instructor was on leave, and the course did contain additional activities during class time. This sample set may not be reliable as a result of this additional variable, but data, nonetheless, were collected and are reported within the flipped group.
Flipped class details
Lecture slides adapted from the publisher were used to instruct both the flipped and non-flipped groups. All sections of the Organic Chemistry I course had three in-class 75 minute exams and a two-hour comprehensive final. For the non-flipped group, the lectures were presented in-class, with periods of group work interspersed between new concepts. For the flipped group, the lectures were assigned as pre-recorded videos to view before attending the class. Both groups were introduced to in-class quizzes about 1 time per week, and homework (optional Fall 2011 and required Spring 2012 to present) was also assigned. The flipped class had additional quizzes completed through the course management system, Blackboard, due several hours before class.Additionally, “review” videos were created before each exam. In the review videos the instructor went through every problem that had been posed in the lectures and worked them out on-screen, as a separate exam review. Because the primary lectures introduced several “try this at home” problems for the students, and the answers were not readily available during class, the review videos focused on solving all the extra problems, such as nomenclature or mechanism examples. These review videos were made available following the completion of all lectures leading up to the exam (i.e. a few days before the exam). Not including the review lectures, the average video length was ∼20 minutes.
After watching the recorded lecture for the appropriate class period, please provide at least one question that you have about the material. Alternatively, you can list your “favorite” or most interesting part of the material. This question can be answered below, OR turned in on paper at the beginning of class (when it is due). The warm-up will be considered incomplete (no credit) if this question is incomplete.
The warm-ups were graded as completion with no make-ups. These quizzes acted as formative assessments and were graded as complete or incomplete. The results of these quizzes guided the content for the beginning of the following class period, much like a just-in-time teaching approach.
Entrance and exit surveys
An exit survey was given to students, in only the flipped classes, at the close of the semester. After flipping the class for two semesters, an additional entrance survey was given to the students on the first day of class. In order to capture the largest audience, surveys were given on paper at the end of a class period while the instructor waited outside the classroom. Each student was informed that participation in the survey(s) was completely voluntary and anonymous and would not affect his or her grade. The instructor did not view the responses to the surveys until after grades for the semester were reported.The exit survey was adapted from an existing survey (Butzler, 2012) and asked approximately 25 questions pertaining to study habits, interactions with the material outside of class, effectiveness of the flipped classroom, and overall student learning. The questions were scored on a scale of 1–5, with 1 being strongly disagree and 5 being strongly agree. In addition to the scaled questions, the survey also included several open-response questions. The most recent set of entrance and exit survey questions (Spring 2014) can be found in Appendix II.
Results and discussion
Grades
The “non-flipped group” contained three non-flipped Organic I classes: Fall 2011 (two sections) and Spring 2012 (one section). The “flipped group” contained three flipped Organic I classes (one section each): Fall 2012, Spring 2013, and Fall 2013. Both of these non-flipped and flipped groups attempted in-class exams, which were the majority of the overall course grade (∼40%). Although the same exact exams were not administered every semester, the content and variety of questions stayed the same. When comparing the non-flipped group and the flipped group, the overall grade distribution appeared to change (Fig. 1). The percentage of students earning a 3.5 (A−/B+) decreased in the flipped group, but the percentage of students earning a 3.0 (B) nearly doubled. The percentage of students earning a 4.0 (A) also increased in the flipped group. The grade of a “W” indicates an official withdrawal from the course. Before the flip, six students withdrew from the course (10%). After implementing the flip, only three students withdrew from the course (4%), while one earned a 0 (F). Of note, the only “0” grade in the flipped group was a student that had to remain in the class to maintain full-time status, but stopped attending class a little after mid-semester.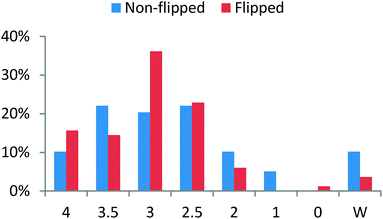 | ||
| Fig. 1 A comparison of the grade distribution, as a percentage of students earning each grade, for the non-flipped group (three sections, n = 59) and the flipped group (three sections, n = 83). | ||
Generally speaking, the grade distribution may indicate that students in the flipped Organic I classroom tend to stick around for the entire semester and not withdraw from the course. For example, if one assumes that these students are weaker in terms of academic abilities, and these students are increasingly sticking with the course until the end, then the overall course grade average might be lowered. If weaker students remain, they are not “weeded out”, but instead are supported until the end. This, arguably, is one of the main goals of using the flipped classroom as a pedagogy—to help those students who might otherwise drop the course. The grade distribution reported in Fig. 1 can be interpreted to show that students were not negatively affected by the flip. The very remarkable students still did very well, while weaker students were more able to successfully complete the course. The increased possibility of weaker students passing the course may help paint the dreaded Organic Chemistry class in a better, more achievable, light. Perhaps the stigma about the course could be lessened by explaining to students that with the flip, success is certainly possible.
In an effort to check that the students in the flipped class were not at a disadvantage compared to those in other concurrent sections that were not flipped, an analysis of student performance at the end of the full organic sequence (after the Organic II course) was conducted, using the summative ACS final exam. Students taking the ACS exam could have experienced varying levels of the flipped class, from zero to two semesters of exposure. Students participated for one semester of the flip (either Organic I or Organic II), two semesters (both Organic I and Organic II), or zero semesters (taking Organic I and Organic II with another instructor). The average exam scores were nearly identical for those students in the flipped class for 1 semester, 2 semesters, or 0 semesters, and were on par with previous semesters (data not shown).
Surveys
Highlighted partial exit survey results are summarized in Table 1. The scale for these questions is 1–5, with 1 being “strongly disagree” and 5 being “strongly agree”. All entrance and exit survey response means are reported in Tables 3 and 4 (Appendix II). Overall, the sample survey responses in Table 1 are positive. For the questions highlighted, all the response averages are on the “agree” end of the spectrum and above a 3.0 (which is neutral). Student responses can be summarized by one particular exit survey comment:| Sample of questions from exit survey | Fall 2012 n = 37 | Spring 2013 n = 22 | Fall 2013 n = 12 | Spring 2014 n = 16 |
|---|---|---|---|---|
| I think that having lectures recorded benefitted me as a student. | 4.30 | 3.59 | 3.73 | 4.19 |
| During class exercises, my problem-solving skills were developed. | 4.32 | 4.23 | 4.36 | 4.47 |
| Listening to lectures outside of the classroom and problem-solving in class is effective. | 4.41 | 3.86 | 4.00 | 4.31 |
| Listening to lectures at home and problem-solving in class was more effective than if I had listened to a lecture during class and did problems on my own at home. | 4.33 | 3.55 | 3.73 | 4.25 |
| Working on problems in class increased my problem-solving comfort level. | 4.46 | 4.36 | 4.18 | 4.50 |
“I LOVE the course format. I must say that it took some time getting used to not having lecture in the classroom, but it grows on you…By coming to class already with material in the back of your head, it definitely makes for a more productive class and any students that are having problems, it seems to help them out as well.”
Towards the end of the semester, students were able to establish a routine. They knew what they needed to do each night to prepare for class, and many were comforted by knowing any questions they had would be answered during the mini-lecture in class. The students in the flipped class knew they could study for exams by reviewing the lectures, and they knew that the problems solved in class were very similar to those they would encounter on exams. Students really embraced the method in the end because they could see how it was benefitting them—something that can be difficult to comprehend at the start of the semester when the method is completely new.
Though the method was successful overall, in terms of student grades trending upwards and student perceptions (exit survey) being positive (high responses toward the “agree” end of the scale), most students opposed the format in the beginning of the semester. When asked the open-ended question, “What do you think of the idea of the “inverted” method of teaching this course?” on the entrance survey (when given), student responses were along the lines of:
“I think it may turn out well in the end, but so far it has been tough getting used to.”
“Right now I think it is tricky and hard, but it has only been 2 lectures.”
Responses to this question have increasingly improved in attitude as the course has been taught in the flipped format. Some of the students in the Spring 2014 semester responded to the same question with:
“Awesome idea, I expect to do well in this course because of this method.”
“I think it will be helpful since things often make sense in lecture but questions arise when trying to apply concepts.”
“I think it will help to just solve problems [in class] and ask questions rather than lecture.”
The continued support and encouragement from the instructor, as well as an almost contagious positive attitude by the instructor on the first day of class during the “buy-in” period may be aiding the increasingly positive outlook by the students.
Although not considered at the onset of utilizing the flipped class method for this organic chemistry course, an additional research question was identified after one year of implementing the flip: “Do students take more ownership of their learning while in the flipped classroom?” By the time students are sophomores they have generally become used to the passive lecture format, and their learning is no longer a self-sufficient journey as a result of the lecture “crutch”. It is important that students, as they grow and mature and become self-sufficient adults in their respective careers, learn to take ownership of their learning. Coming to class and writing down exactly what the instructor writes on the board is, for the most part, easy; taking that material and using it to solve problems is not. At the beginning and end of three semesters (Spring and Fall 2013, and Spring 2014) students in the Organic Chemistry I course were surveyed. Comparative data between the start of the semester (entrance) and the end of the semester (exit) are available for three questions regarding student learning, asked on both surveys (Table 2).
| Spring 2013 entrance (n = 24) | Spring 2013 exit (n = 22) | p | Fall 2013 entrance (n = 10) | Fall 2013 exit (n = 12) | p | Spring 2014 entrance (n = 21) | Spring 2014 exit (n = 16) | p | |
|---|---|---|---|---|---|---|---|---|---|
| a Values are statistically different from entrance to exit (p < 0.05). b Values in parentheses denote one standard deviation. | |||||||||
| I feel confident in my organic chemistry problem-solving skills. | 2.33 (1.14)b | 3.50 (0.86) | 0.00169 | 3.44 (1.01) | 3.50 (0.50) | 0.760 | 2.71 (1.11) | 3.69 (0.70) | 0.00433 |
| I have a good understanding of organic chemistry, and feel comfortable explaining concepts to others. | 1.71 (0.91) | 2.38 (1.32) | 0.0507 | 2.50 (0.85) | 3.58 (0.64) | 0.00320 | 2.19 (1.17) | 3.63 (0.81) | <0.001 |
| I feel autonomous in my learning. (That is, I am in control of what I learn or don't learn) | 4.48 (0.68) | 4.44 (0.81) | 0.876 | ||||||
For the first two questions, the responses increased towards “strongly agree” at the end of the semester. This increase indicates that students were, in fact, becoming more comfortable with the material as the semester came to a close. This result is not surprising given the fact that the entrance survey is generally distributed on the first day or two of class when the students have almost no organic chemistry background. Although not statistically significant, the last question in Table 2 was asked in only one semester so far, and the result was not one that was anticipated. Students at the start of the semester reported that they were more in control of their own learning (response of 4.48) than at the end of the semester (4.44). This unexpected result could be explained by both the small sample size, and the fact that only 16 of the original 21 students completed the exit survey (several were absent that day). Additionally, since this was the first time that question was asked on the survey, it will be important to continue to ask that same question and follow-up with the original theory: students will feel more in control of their success in the classroom (learning) as the semester progresses in a flipped class.
Instructor's observations
From a more qualitative perspective, the instructor's observations can be used to report on the effectiveness of the practice of flipping the classroom. One such observation was during the first flipped semester (Fall 2012), when the instructor witnessed an argument about mechanistic arrows. The class was working on a problem that involved synthesizing information based on a concept to which the students had just been introduced. They recently learned how to draw curved mechanistic arrows, and were facing a problem where the arrows were missing from a mechanism, but the reactants and products were drawn. The particular problem at hand involved a carbonyl reaction, and since this chemistry is discussed in the second semester portion of the course, the students were truly synthesizing their own answers. Two students in a group of four felt very strongly that they had the correct arrows drawn for the given set of products, while the other two students in the group felt very strongly that THEY had the correct answer. The group called in the instructor to settle the dispute, and after both sides giving testimony, the correct answer was revealed by the instructor. The students were extremely passionate and lively in their discussion—something that was not observed in the more lecture-based course.For this course, each student was expected to report (written or oral) the answers to problems regularly throughout the semester. One natural outcome of the flipped class is that students become increasingly comfortable with sharing answers and discussing them as a large group. This outcome was more visible compared to the lecture-based classroom, mostly because the non-flipped class did not allow enough time to have rich discussions and debates about several topics during each class period.
The format of the flipped class allowed for deeper learning, critical thinking, and problem solving during the reclaimed class time where the instructor was face-to-face with the students. For this study, the method of problem-solving did not differ much in the flipped class from the non-flipped class; however, the amount of time available to cover additional problems and discuss them thoroughly increased with the flip. This extra time that the instructor spent with the students is crucial, as the instructor interacted with every single student during every single class period—something unheard of in the lecture format (taught by the same instructor). Interestingly, increased success of weaker students could be attributed to the instructor's ability to individualize the course: chat with each student, hold each of them accountable for preparing for class, and inquire about where they might have additional problems or questions.
Potential problems
One problem that persisted with the course format is the inability of students to ask questions immediately as they see and hear content for the first time. Students were instructed to take notes, write down questions, and save questions for in-class discussions. Additionally, questions could be submitted by email or by answering the warm-up quiz question (“muddiest point”). An online forum is one way to encourage student questions, although many students are too nervous to ask questions in a public setting (and one that can be tracked with their name). One way to circumvent the inability to ask immediate questions is to offer students instant feedback in the form of a text message. This option was available to the Spring 2014 class; surprisingly no students utilized it.An additional problem with the flipped format is the inability of the instructor to know if each student viewed each lecture in its entirety. For the flipped classroom to work smoothly, each student must come to class prepared. Whether the student watched the lecture video, read the book, or watched YouTube videos on the topic, it does not particularly matter, as long as he or she had first exposure to the content before arriving to class. Without a way to check for viewing of the lecture videos, students were not held accountable for their preparation, aside from the warm-up quiz (completion) and interactions with the instructor during the allotted participation time. In most cases students who failed to prepare for class had a legitimate reason and did not form a habit of avoiding the lecture videos. Students felt guilty when they could not participate and help within their groups. To address this issue in the future, a set of notes could be required (and graded) to ensure first exposure to content outside of class.
Conclusions
As with any new technique or pedagogical tool, there is no one solution to every problem, and the decision to flip a class, especially a gateway course to other required courses, is not one to take lightly. The format of this course was changed dramatically when all of the content was moved from during class time to outside of class as homework. It is important to recognize that this technique will not be useful for every organic chemistry course, instructor, or class of students, but in this case, the student performances and responses were positive.One reason for the success of the flip in this particular setting could be that the student buy-in was successful at the start of the semester. The instructor made a pointed effort to be very positive about the format, stressing to the students the reasons why it would be beneficial to them. Some of those beneficial attributes of the course that were communicated to the students include: the ability to pause, rewind, and re-watch the lecture videos—something that is not possible with in-class “live” lectures; lectures available for review for the entire semester; and recorded review lectures as an option to summarize and practice the material before exams. Another important point made to the students was that they could be completely in control of their own learning. They could take advantage of all the resources available to them (i.e. re-watch lectures, work many problems in class, practice at home after already practicing in class, etc.) and in the end, should feel satisfied with a job well-done. The initial semester the flip was introduced, the students were told that it was a new technique and the instructor would re-visit the technique as the semester progressed, in case the whole thing was a flop. Mid-semester evaluations were collected and the response was >90% positive from the group, so the format continued. Once that semester was complete, the instructor was forthcoming with the improvement in student grades from non-flipped to flipped. If students thought they were more likely to earn an A, not that it was easier to earn an A, they were on board. The grade improvement results alone were enough to help get subsequent sections of the course on board with the method.
In summary, the flipped classroom appears to be an effective pedagogical approach to teaching organic chemistry in a small college setting. Student performance (as measured by grades) appeared to be the same or improved with the flipped format, and student attitudes toward the flipped class were positive, with 70–90% of each class liking the format. Students' comfort level in solving problems increased over the course of a flipped semester and their confidence in the material improved. Students were exposed to difficult content outside of class, at home, where they could pause and re-watch parts of the lecture, take notes at their own pace, and watch review lectures before exams. Class time, then, was used for answering questions and applying the content, which in a more lecture-based classroom is usually reserved for homework and office hours. With the flipped method students can take more responsibility for their learning in an active way and be more aware of what they are capable of learning.
Appendix I: topics per video (24) for the Organic Chemistry I course
1. Lecture_01_Ch1: Remembering General Chemistry: Electronic structure and bonding (30 min)2. Lecture_02_Ch2: Acids and Bases: Central to understanding organic chemistry (19 min)
3. Lecture_03_Ch2: Chapter 2, continued; Resonance revisited (17 min)
4. Lecture_04_Ch3: An Introduction to Organic Compounds (alkane nomenclature) (28 min)
5. Lecture_05_Ch3: Newman projections (25 min)
6. Lecture_06_Ch3: Cyclohexane and chair conformations (30 min)
7. Lecture_07_Ch14: Infrared Spectroscopy (23 min)
8. Lecture_08_Ch14: IR continued: annotating spectra; determining unknowns (11 min)
9. Lecture_09_Ch14: Mass Spectrometry (10 min)
10. Lecture_10_Ch4: Isomers: The arrangement of Atoms in space (28 min)
11. Lecture_11_Ch4: Chapter 4, continued (18 min)
12. Lecture_12_Ch5: Alkenes: Structure, Nomenclature (17 min)
13. Lecture_13_Ch5: Alkenes: Introduction to Reactivity, thermodynamics and kinetics (19 min)
14. Lecture_14_Ch6: Reactions of Alkenes; Mechanisms (25 min)
15. Lecture_15_Ch6: Stereochemistry of Addition reactions (20 min)
16. Lecture_16_Ch8: Delocalized electrons and their effect on stability (17 min)
17. Lecture_17_Ch8: Diels Alder reactions (25 min)
18. Lecture_18_Ch8: Thermodynamic and Kinetic products (reactions of dienes) (17 min)
19. Lecture_19_Ch7: The reactions of alkynes (18 min)
20. Lecture_20_Ch7: Reactions of alkynes, continued; Synthesis (19 min)
21. Lecture_21_Ch9: Substitution reactions (SN2) (23 min)
22. Lecture_22_Ch9: Substitution reactions (SN1); Comparing SN1 and SN2; Synthesis (15 min)
23. Lecture_23_Ch10: Elimination reactions of alkyl halides (18 min)
24. Lecture_24_Ch10: Competition between substitution and elimination; Synthesis (22 min)
Appendix II: entrance and exit surveys
Part 1. Survey questions
1. I have had recorded lectures available to me in courses prior to this one.
2. I think that having lectures recorded will benefit me as a student.
3. I feel confident in my organic chemistry problem-solving skills.
4. I feel autonomous in my learning. (That is, I am in control of what I learn or don't learn)
5. I am nervous about the format of this course because I have never done anything like this before.
6. I think that problem-solving in class will help me practice problem-solving outside of class and on exams, homework assignments, and quizzes.
7. I have a good understanding of organic chemistry, and feel comfortable explaining concepts to others.
Open-ended questions:
What do you think of the idea of the “inverted” method of teaching this course?
What in-the-class methods are usually helpful for you in learning chemistry?
What out-of-the-class methods are usually helpful for you in learning chemistry?
What are your current study habits with respect to chemistry or science courses?
1. I interacted with the instructor at least 4 times during the traditional class meeting.
2. I interacted with the instructor at least 1 hour per week outside of the traditional class meeting.
3. I have had recorded lectures available to me in courses prior to this one.
4. I think that having lectures recorded benefitted me as a student.
5. When I watched the recorded lectures I watched and listened.
6. When I watched the recorded lectures I took some notes.
7. When I watched the recorded lectures I wrote down questions.
8. When I watched the recorded lectures I paused and rewound the video.
9. During class exercises, my problem-solving skills were developed.
10. I feel autonomous in my learning. (That is, I am in control of what I learn or don't learn)
11. When I left the class, I felt that I could do problems on my own.
12. When I left the class I attempted to do problems outside of class.
13. Working on problems in class increased my problem-solving comfort level.
14. Listening to lectures outside of the classroom and problem-solving in class is effective.
15. I feel confident in my organic chemistry problem-solving skills.
16. I think that problem-solving in class has helped me practice problem-solving outside of class and on exams, homework assignments, and quizzes.
17. Listening to lectures at home and problem-solving in class was more effective than if I had listened to a lecture during class and did problems on my own at home.
18. I would take another class if it was “inverted”.
19. The warm-up exercises and discussion helped me focus on the key concepts within each chapter of the text.
20. I have a good understanding of organic chemistry, and feel comfortable explaining concepts to others.
21. I am still nervous about the format of this course, even at the end of the semester.
22. How often did you watch the pre-recorded lectures? (can circle more than one) never once or twice per semester when I was absent before tests or quizzes once per month once or twice per week once per day.
Open-ended questions:
What did you think of the “inverted” method of teaching this course? Did your opinion change as the semester progressed?
What in-the-class methods did you find especially helpful in this course?
What out-of-the-class methods did you find especially helpful in this course?
How did your study habits change in the inverted classroom structure, over the course of the semester, if at all?
Part 2. Survey response data
| Entrance survey questions | Spring 2013 n = 24 | Fall 2013 n = 10 | Spring 2014 n = 21 | |||
|---|---|---|---|---|---|---|
| Mean | Standard deviation | Mean | Standard deviation | Mean | Standard deviation | |
| a Question 4 was added in Spring 2014 only. | ||||||
| Q1 | 1.96 | 1.49 | 2.00 | 1.63 | 2.71 | 1.55 |
| Q2 | 3.92 | 1.06 | 4.30 | 0.82 | 4.30 | 0.86 |
| Q3 | 2.52 | 1.08 | 3.40 | 0.97 | 2.76 | 1.04 |
| Q4a | 4.48 | 0.68 | ||||
| Q5 | 3.00 | 1.10 | 2.44 | 1.24 | 2.71 | 1.38 |
| Q6 | 4.29 | 0.69 | 4.40 | 0.70 | 4.48 | 0.60 |
| Q7 | 1.71 | 0.91 | 2.50 | 0.85 | 2.19 | 1.17 |
| Entrance survey questions | Fall 2012 n = 37 | Spring 2013 n = 22 | Fall 2013 n = 12 | Spring 2014 n = 16 | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | Standard deviation | Mean | Standard deviation | Mean | Standard deviation | Mean | Standard deviation | |
| a Question 10 was added in Spring 2014. b Question 16 in 2012 and 2013 read: “I changed my study habits after the first exam”. The question 16 reported on above replaced the previous statement in Spring 2014. Responses to the previous version of question 16 are not included. | ||||||||
| Q1 | 4.08 | 1.01 | 3.95 | 1.17 | 4.50 | 0.52 | 4.38 | 0.81 |
| Q2 | 2.22 | 1.27 | 1.73 | 0.88 | 1.50 | 0.52 | 2.69 | 1.20 |
| Q3 | 1.59 | 1.28 | 2.32 | 1.78 | 1.67 | 1.37 | 1.69 | 1.49 |
| Q4 | 4.30 | 1.10 | 3.59 | 1.40 | 3.58 | 1.24 | 4.19 | 0.98 |
| Q5 | 4.41 | 0.80 | 4.23 | 1.15 | 4.25 | 0.97 | 4.19 | 0.83 |
| Q6 | 4.46 | 0.84 | 4.27 | 1.08 | 3.50 | 1.31 | 4.50 | 0.73 |
| Q7 | 2.78 | 1.23 | 3.27 | 1.39 | 2.75 | 1.14 | 3.00 | 1.21 |
| Q8 | 4.57 | 0.69 | 4.18 | 1.14 | 4.50 | 0.67 | 4.69 | 0.70 |
| Q9 | 4.32 | 0.88 | 4.23 | 0.87 | 4.33 | 0.89 | 4.47 | 0.64 |
| Q10a | 4.44 | 0.81 | ||||||
| Q11 | 4.00 | 0.94 | 3.86 | 1.13 | 3.75 | 1.06 | 3.88 | 0.81 |
| Q12 | 4.00 | 1.05 | 4.00 | 0.87 | 3.42 | 1.00 | 4.19 | 0.98 |
| Q13 | 4.46 | 0.84 | 4.36 | 0.73 | 4.08 | 0.79 | 4.50 | 0.63 |
| Q14 | 4.41 | 0.72 | 3.86 | 1.17 | 3.75 | 1.22 | 4.31 | 1.01 |
| Q15 | 3.89 | 0.97 | 3.50 | 0.86 | 3.50 | 0.52 | 3.69 | 0.70 |
| Q16b | 4.50 | 0.63 | ||||||
| Q17 | 4.33 | 1.01 | 3.59 | 1.53 | 3.50 | 1.17 | 4.25 | 1.13 |
| Q18 | 4.00 | 1.18 | 3.55 | 1.44 | 3.83 | 0.94 | 4.19 | 1.05 |
| Q19 | 3.59 | 1.07 | 3.86 | 0.99 | 3.75 | 0.87 | 4.06 | 1.12 |
| Q20 | 3.92 | 1.12 | 3.50 | 1.10 | 3.58 | 0.67 | 3.63 | 0.81 |
| Q21 | 1.95 | 1.31 | 2.38 | 1.32 | 2.67 | 1.15 | 2.56 | 1.31 |
Notes and references
- Arnaud, C. H., (2013), Flipping Chemistry Classrooms, Chem. Eng. News, 91, 41–43.
- Bergmann, J. and Sams, A., (2012), Flip Your Classroom: Reach Every Student in Every Class Every Day, Washington, DC: International Society for Technology in Education.
- Bradley, A. Z., Ulrich, S. M., Jones Jr., M. and Jones, S. M., (2002), Teaching the Sophomore Organic Course without a Lecture. Are You Crazy? J. Chem. Educ., 79, 514–519, DOI: http://10.1021/ed079p514.
- Butzler, K., (2012), Survey questions for the inverted class, Retrieved from http://kellybutzler.wikispaces.com/ (accessed May 29, 2012).
- Christiansen, M. A., (2014), Inverted Teaching: Applying a New Pedagogy to a University Organic Chemistry Class. J. Chem. Educ., 91, 1845–1850, DOI: http://10.1021/ed400530z.
- Crouch, C. and Mazur, E., (2001), Peer Instruction: Ten years of experience and results, Am. J. Phys., 69, 970–977, DOI: http://10.1119/1.1374249.
- Davies, R. S., Dean, D. L. and Ball, N., (2013), Flipping the classroom and instructional technology integration in a college-level information systems spreadsheet course, Educ. Technol. Res. Dev., 61, 536–580, DOI: http://10.1007/s11423-013-9305-6.
- Foertsch, J., Moses, G., Strikwerda, J. and Litzkow, M., (2002), Reversing the Lecture/Homework Paradigm Using eTEACH Web-based Streaming Video Software, J. Eng. Educ., 267–274, DOI: http://10.1002/j.2168-9830.2002.tb00703.x.
- Halloun, I. A., (1985), The initial knowledge state of college physics students, Am. J. Phys., 53, 1043–1048, DOI: http://10.1119/1.14030.
- Lage, M. J., Platt, G. J. and Treglia, M., (2000), Inverting the Classroom: A Gateway to Creating an Inclusive Learning Environment, J. Econ. Educ., 30–43, DOI: http://10.2307/1183338.
- Lasry, N., Mazur, E. and Watkins, J., (2008), Peer instruction: From Harvard to the two-year college, Am. J. Phys., 76, 1066–1069, DOI: http://10.1119/1.2978182.
- McLaughlin, J. E., Roth, M. T., Glatt, D. M., Gharkholonarehe, N., Davidson, C. A., Griffin, L. M., Esserman, D. A. and Mumper, R. J., (2014), The Flipped Classroom: A Course Redesign to Foster Learning and Engagement in a Health Professions School, Acad. Med., 89, 236–243, DOI: 10.1097/ACM.0000000000000086.
- Pierce, R. and Fox, J., (2012), Vodcasts and Active-Learning Exercises in a “Flipped Classroom” Model of a Renal Pharmacotherapy Module, Am. J. Pharm. Educ., 76(10), Article 196, 1–5, DOI: http://10.5688/ajpe7610196.
- Prince, M., (2004), Does Active Learning Work? A Review of the Research, J. Eng. Educ., 93, 223–231, DOI: http://10.1002/j.2168-9830.2004.tb00809.x.
- Seymour, E. and Hewitt, N. M., (1997), Talking About Leaving: Why Undergraduates Leave the Sciences, Boulder, CO: Westview Press.
- Smith, J. D., (2013), Student attitudes toward flipping the general chemistry classroom, Chem. Educ. Res. Pract., 14, 607–614, DOI: 10.1039/c3rp00083d.
- Strayer, J. F., (2012), How learning in an inverted classroom influences cooperation, innovation and task orientation, Learn. Environ. Res., 15, 171–193, DOI: http://10.1007/s10984-012-9108-4.
- Teo, T. W, Tan, K. C. D., Yan, Y. K., Teo, Y. C. and Yeo, L. W., (2014), How flip teaching supports undergraduate chemistry laboratory learning, Chem. Educ. Res. Pract., 15, 550–567, DOI: 10.1039/C4RP00003J.
| This journal is © The Royal Society of Chemistry 2015 |
