The logical and psychological structure of physical chemistry and its relevance to the organization/sequencing of the major areas covered in physical chemistry textbooks
Georgios
Tsaparlis
Department of Chemistry, University of Ioannina, GR-451 10 Ioannina, Greece. E-mail: gtseper@cc.uoi.gr
First published on 15th April 2014
Abstract
Jensen's scheme for the logical structure of chemistry is taken as reference to study the logical structure of physical chemistry. The scheme distinguishes three dimensions (composition and structure, energy, and time), with each dimension treated at one of the three levels (molar, molecular, and electrical). Such a structure places the outer limits, leading to two alternative approaches to teaching physical chemistry: the ‘analytical approach’, which reflects the historical development of the field, starts at the molar level with matter (substances as we find them in the laboratory), and moves to the molecular and electrical levels; and the ‘synthetic approach’, which begins with the structure and behavior of matter. An analysis of twenty physical chemistry textbooks was carried out with the aim of examining the organization/sequencing in the books of the major areas of physical chemistry. A dichotomy between the macroscopic/phenomenological and the submicroscopic molecular/atomic/electronic approaches to physical chemistry is evident from the analysis. Although phenomenological subjects are also abstract, involving complicated concepts and mathematics, quantum chemistry and statistical thermodynamics are considered to be more difficult. Most authors favor the traditional analytical approach, while others prefer to focus on the molecular synthetic approach. However, many authors remain open to alternative approaches of their own.
Introduction
The German scientist Wilhelm Ostwald (1853–1932), who won the Nobel prize in chemistry in 1909 for his work on catalysis, chemical equilibria, and reaction velocities, is considered as the cofounder (Lagowski, 2004), and even the ‘father’ of modern physical chemistry (Sutton, 2003). The Dutch physical chemist Jacobus van't Hoff (1852–1911), who received the first Nobel prize in chemistry in 1901 for his work on chemical dynamics and the osmotic pressure in solutions, and the Swedish physical chemist Svante Arrhenius (1859–1927), who received the Nobel prize in chemistry in 1903 for his theory of electrolytic dissociation are generally considered along with Oswald as the cofounders of physical chemistry.Historically, physical chemistry started as a phenomenological discipline. Ostwald devoted much of his life to the study of energy, considering that matter was “a mirage which the mind creates to comprehend the workings of energy” (Sutton, 2003). He insisted that atoms and molecules, being not directly observable, were hypothetical entities, which could just serve as convenient symbols for statistical regularities in our observations, but otherwise there was no place for them in “the basic truths of science, which should be expressed in terms of energetics”. Only, in 1909, did Ostwald finally accept the reality of atoms, and this only after much controversy, having been convinced by new physical evidence (including Jean Perrin's studies of Brownian motion) (Sutton, 2003). Meanwhile, phenomenological physical chemistry made great progress in various fields, such as chemical thermodynamics, electrochemistry, and chemical kinetics. Chemical thermodynamics was introduced by Josiah Willard Gibbs in his famous paper “On the equilibrium of heterogeneous substances” published in 1876. Electrochemistry is assumed to have been founded by Frederick Daniell and Michael Faraday. Chemical kinetics was developed by the formulation of the law of mass action in 1864 by Peter Waage and Cato Guldberg.
Another important area of physical chemistry is statistical thermodynamics (a branch of statistical mechanics), which culminated with the statistical interpretation of the second law of thermodynamics by Ludwig Boltzmann with his 1877 paper entitled “On the relation between the second law of the mechanical theory of heat and the probability calculus with respect to the theorems on thermal equilibrium” (Flamm, 1997). J. Willard Gibbs also contributed greatly to the development of statistical mechanics, a term that he coined.
The foundations of quantum theory were laid in the early 1900s (Pilar, 1968, ch. 1), with the contributions of (among others) Max Planck (in 1901), Albert Einstein (in 1905), Ernest Rutherford (in 1911) and Niels Bohr (in 1913). These advancements culminated in Louis de Broglie's wave model of particles (in 1924), and in Werner Heisenberg's (in 1925) and Erwin Schrödinger's (in 1926) development of quantum and wave mechanics respectively.
Today, scientists agree that quantum mechanics is the cornerstone of chemistry, including physical chemistry. In 1927, the Heitler–London valence-bond model enabled the quantum mechanical calculation of bonding properties of the hydrogen molecule and laid the foundations of quantum chemistry. These and other developments convinced Paul Dirac to state in 1929 that: “The general theory of quantum mechanics is now complete… The underlying physical laws necessary for the mathematical theory of a large part of physics and the whole of chemistry are thus completely known” (Dirac, 1929, p. 714).
The reflection of the history of physical chemistry in physical chemistry textbooks
As noted above, by 1913, the foundations of quantum theory had been laid with the Bohr model of the atom. It comes then as no surprise that in the same year the first edition of Frederick Getman's textbook “Outlines of Theoretical Chemistry”, in which the last four chapters represented a remarkable and ambitious attempt to treat the then modern topics of photochemistry, the electrical theory of matter, radioactivity and atomic structure, was published (Getman, 1913). Meanwhile the book had started with topics well-established at the time, such as gases, liquids, and solids, thermodynamics, solutions, electrolytes, colloids, thermochemistry, equilibria, kinetics, and electrochemistry.In 1935, the first book on quantum chemistry was written by Pauling and Wilson (1935), possibly leading Samuel Glasstone to incorporate in his “Textbook of physical chemistry” “Atomic structure and atomic spectra” as the first chapter (Glasstone, 1940). The author justified this with the following statement:
“It has been said that chemistry deals with the combination of atoms … atomic combination involves atomic forces, and it is one of the objects of physical chemistry to see how far the chemical interactions between atoms and molecules can be interpreted by means of the forces existing between and within atoms. It is reasonable, therefore, that a book on physical chemistry should begin, as does the present one, with a consideration of the structure of atoms. An understanding of this subject requires the knowledge of spectra, as well as of quantum theory, and so these topics are presented together with a brief discussion of the fundamentals of wave mechanics” (p. xi).
If one takes a look at most recent and current physical chemistry textbooks, one will note that they begin with a study of thermodynamics, then discuss quantum chemistry, and treat chemical kinetics last. In point of fact, these are the three principal areas of physical chemistry: thermodynamics concerns the energetics of chemical reactions, quantum chemistry concerns the structures of molecules, and chemical kinetics concerns the rates of chemical reactions (McQuarrie and Simon, 1997). For these authors, this sequencing “is a reflection of the historical development of the field” (pp. xvii–xviii); but, as modern PC is based on quantum mechanics, they decided to follow a molecular approach to their own PC textbook.
The present work – research questions
In this work, Jensen's scheme for the logical structure of chemistry is taken as reference to study the logical structure of physical chemistry (PC). This structure is then analyzed in relation to the psychology of learning. With such a rationale, a survey and analysis of the organization/sequencing of the major areas of PC in many PC textbooks will be undertaken, with the aim of identifying the various hierarchies that are adopted by the authors, ranging from traditional analytical/historic approaches to synthetic approaches (the latter based their development on the structure of matter – on quantum chemistry). The main research question is as follows:• What is the connection of the organization/sequencing of the various major areas covered in physical chemistry textbooks with the logical and psychological structure of physical chemistry?
A related research question is:
• What are the arguments that support the various textbook organizations?
To answer the second question, an exposition of textbook authors' philosophies with respect to the structure and sequencing of the various areas will be presented, based on the prefaces of the various books. This exposition constitutes then a necessary complement to the textbook analysis.
The ultimate question of this research program is:
• “Is there an optimum teaching–learning sequence for undergraduate instruction for the various major areas of the subject?”
This question cannot be answered by the analysis of textbooks. Consideration of a wide range of information should be involved. This issue is discussed further in prospects for further work at the end of this paper.
Rationale: the logical structure of physical chemistry and the structure of physical chemistry textbooks
The study of PC is an integral part and a ‘sine qua non’ in the training of chemists. On the other hand, it is perceived as a difficult course. Moore and Schwenz (1992) have commented that this course “sets the tone” of the chemistry major. A number of chemistry and science-education studies have focused on general aspects of teaching and learning PC (Nicoll and Francisco, 2001; Derrick and Derrick, 2002; Hahn and Polik, 2004; Sözbilir, 2004) as well as on the difficulties of student understanding and misconceptions in various areas of PC. For a review see Tsaparlis (2008).Authors of various PC textbooks that are reviewed in this paper (see Table 1) have various views about the difficulty of the subject. Andrews (book #1) verifies that “At many institutions physical chemistry gets the largest number of votes as the most difficult course” (p. vii), while for Vemulapalli (#8) “physical chemistry has a reputation for being fascinating and fearsome. … it cannot be denied that the subject is abstract and requires effort for mastery” (pp. xiv–xv). In the opinion of Vemulapalli, “the uneasiness of many students with the subject is not related to its abstract nature”, but to the fact that many topics in physical chemistry like orbitals, entropy, Gibbs free energy, and magnetic resonance are introduced ahead of the proper sequence and before the student has acquired the necessary background (p. xv). On the other hand, Winn (#6) comments that: “There is a suspicion among students that physical chemistry is the “Great White Whale” of the chemistry curriculum. … but physical chemistry is neither impossibly difficult nor frighteningly challenging” (p. xix). Finally, according to Pratton, Maron and Lando (#16), “Authors of an introductory text must keep … in mind … that the material presented is within the students' grasp. Omission of this precaution leads to student confusion; and instead of interesting and stimulating students in the field, such works frequently develop a revulsion toward the subject which leads to a hasty retreat from it” (p. v).
| Author(s) | Book title | Edition | Year | Publisher | |
|---|---|---|---|---|---|
| a Books #1 and 2 have statistical thermodynamics integrated into thermodynamics. b Book #3 has statistical thermodynamics immediately after phenomenological thermodynamic chapters but before electrochemistry, heterogenous equilibria, and solutions. c Books #7 and #17 do not contain electrochemistry. d Book #11 starts with a chapter on the properties of gases. e Book #19 covers electrolytes but not electrochemical cells. | |||||
| A. Introductory material (e.g. gases and kinetic theory)/thermodynamics/electrochemistry/chemical kinetics/quantum chemistry/additional topics | |||||
| 1 | Donald H. Andrews | Introductory Physical Chemistrya | 1st | 1970 | McGraw-Hill |
| 2 | Arthur W. Adamson | A Textbook of Physical Chemistrya | 3rd | 1986 | Academic Press |
| 3 | Clyde Metz | Schaum's Outline of Physical Chemistryb | 2nd | 1988 | McGraw-Hill |
| 4 | Keith J. Laidler, John H. Meiser, B. C. Sanctuary | Physical Chemistry | Electronic edition | 2010 | Wiley |
| B. Introductory material (e.g. gases and kinetic theory)/thermodynamics/electrochemistry/quantum chemistry/statistical thermodynamics/chemical kinetics/additional topics | |||||
| 5 | Marvin K. Kemp | Physical Chemistry: A Step-by-Step Approach | 1st | 1979 | Marcel Dekker |
| 6 | John S. Winn | Physical Chemistry | 1st | 1995 | Harper Collins |
| 7 | Peter Atkins and Julio de Paula | Atkins Physical Chemistryc | 2009 | Oxford University Press | |
| 8 | G. K. Vemulapalli | Invitation to Physical Chemistry | 1st | 2010 | Prentice-Hall |
| 9 | Gordon M. Barrow | Physical Chemistry | 6th | 1996 | McGraw-Hill |
| 10 | Gilbert W. Castellan | Physical Chemistry | 3rd | 1983 | Addison-Wesley |
| C. Thermodynamics/electrochemistry/quantum chemistry/kinetic theory/statistical thermodynamics/chemical kinetics/additional topics | |||||
| 11 | Lionel M. Raff | Principles of Physical Chemistryd | 1st | 2001 | Prentice Hall |
| 12 | Robert J. Silbey, Robert A. Alberty, Moungi G. Bawendi | Physical Chemistry | 4th | 2005 | Wiley |
| 13 | Thomas Engel and Philip Reid | Physical Chemistry with Mastering Chemistry | 3rd | 2012 | Pearson/Benjamin Cummings |
| D. Thermodynamics/electrochemistry/kinetic theory/chemical kinetics/quantum chemistry/statistical thermodynamics/additional topics | |||||
| 14 | Ira N. Levine | Physical Chemistry | 6th | 2009 | McGraw-Hill |
| E. Placing statistical thermodynamics earlier | |||||
| 15 | John W. Moore | Physical Chemistry | 5th | 1972 | Longman |
| F. Placing quantum chemistry first | |||||
| 16 | Carl F. Pratton, Samuel H. Maron, Jerome B. Lando | Fundamentals of Physical Chemistry | 3rd | 1974 | Macmillan |
| 17 | Donald A. McQuarrie, John D. Simons | Physical Chemistry – A Molecular Approachc | 1st | 1997 | University Science Books |
| 18 | Mark Ladd | Introduction to Physical Chemistry | 3rd | 1998 | Cambridge University Press |
| 19 | R. Stephen Berry, Stuart A. Rice, John Ross | Physical Chemistrye | 2nd | 2000 | Oxford University Press |
| 20 | Hans Kuhn, Horst-Dieter Fösterling, David H. Waldeck | Principles of Physical Chemistry | 2nd | 2009 | Wiley |
It is generally accepted that the role of textbooks is crucial in the teaching process.† According to modern views, teaching can be considered as a special form of communication and interpersonal relationships between the teacher and the students. The textbook supports this communication and contributes more to the growth of interpersonal relationships in the classroom than other teaching tools, such as transparencies, experiments, videoes, personal computers, etc. (Armbruster and Anderson, 1991). As a consequence, teachers rely, depend on, closely follow, and use textbooks in their everyday practice (Britton et al., 1993). The science education literature has considered the important role that is played by the textbooks in the science classroom, although the studies reported deal mainly with elementary and secondary education. According to Chiappetta and Fillman (2007), in the majority of cases, the textbooks become the science curriculum and determine the content of teaching and learning, being used as the primary organizers of subject matter. Also, Weiss et al. (2003) found that textbooks exert the second most important influence on the taught content, following state and district standards/requirements. Finally, Kahveci (2010) has carried out a quantitative analysis of science and chemistry textbooks for indicators of reform.
Of special interest in this work is knowledge organization in PC textbooks, as this is reflected in the order of presentation of the various areas of the subject. Knowledge organization is a critical contributor to effective teaching and learning (Novak, 1990; diSessa, 1993; Ball and Cohen, 1996; Davis and Krajcik, 2005). Consequently, textbook organization is vital for providing insights and roadmaps that could be adopted in course organization. As university lecturers are less bound by state regulations and have freedom in their adoption of textbooks, an in-depth understanding of the knowledge organization in PC textbooks will assist them in adopting a teaching hierarchy and in making their textbook choices.
The logical and psychological structure of physical chemistry
Of particular relevance to this work is Jensen's scheme for the logical structure of chemistry (Jensen, 1998). He distinguishes three dimensions: D1: Composition and structure; D2: Energy; D3: Time. Each dimension can be treated at one of the three levels: L1: Molar level; L2: Molecular level; L3: Electrical level. This scheme leads to the distinction of 3 × 3 = 9 subdivisions, of which the simplest is the treatment of composition at the molar level (D1L1) (see Fig. 1). Phenomenological thermodynamics is at the energy dimension/molar level (D2L1), quantum chemistry is at the structure dimension/electrical level (D1L3), and statistical thermodynamics is at the energy dimension/electrical level (D2L3). The treatment of chemical kinetics at the electrical level is the most complicated case (D3L3).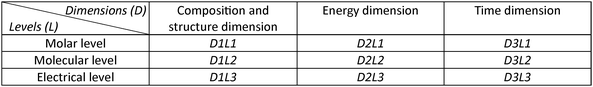 | ||
| Fig. 1 The scheme exhibiting the logical structure of chemistry according to Jensen (1998). | ||
Such a structure places the outer limits, leading to two alternative forms of ‘logic’, and hence two alternative approaches to teaching PC (Moore, book #15, Table 1): the ‘analytical approach’ and the ‘synthetic approach’ (p. 3). A central pedagogical question which arises is if one or the other approaches should be preferred in teaching. The psychology of learning can help us in attempting to answer this question.
Structural concepts from different perspectives of science education
The variation of dimensions and levels in Jensen's scheme makes it imperative to consider the position of science education research on the molecular and electrical levels. Of particular relevance in our case are the concepts of quantum mechanics and statistical thermodynamics. Research has shown that the structural concepts of matter are ill understood and result in great conceptual difficulties among students at all levels of education (Tsaparlis and Sevian, 2013). The difficulties can be explained by examining the relevant concepts from different perspectives of science education, such as (i) the Piagetian developmental perspective, (ii) the Ausubelian theory of meaningful learning, (iii) the information-processing theory, and (iv) the alternative conception movement (Tsaparlis, 1997). According to Herron (1978), abstract concepts such as atom and molecule (which have imperceptible examples and imperceptible attributes) should be considered formal in the Piagetian sense; hence, it is quite likely that they cannot be totally understood without some formal reasoning. From the Ausubelian meaningful learning, as well as from the information-processing perspective, Johnstone (1991, 2000) has argued that structural models take a long time to grow, but once we have embedded them in long-term memory, we can use them as a powerful way of looking at the world (Johnstone, 1991). On the other hand, teaching models and approaches are partially responsible for the existence of a common alternative conceptual framework about the structural concepts, because “The theoretical world of molecules, ions and electrons is not directly available to learners, so alternative conceptions are unlikely to be formed either by direct abstraction from experience, or by acquiring folk-knowledge (as talk about molecular structure and the like is seldom part of everyday life-world discourse)” (Taber, 2013, p. 411).The major conceptual problem of quantum mechanics is that it is fundamentally different from classical physics. It has even been suggested that it requires thinking abilities beyond Piagetian formal operations for an adequate understanding of quantum-mechanical (and relativistic) issues (Castro and Fernandez, 1987). These post-formal operations include what has been termed as quantum logic (Birkoff and von Newmann, 1936). However, the above analysis and discussion do not imply that the treatment of the various areas of PC at the phenomenological/molar level is simple or does not cause its own difficulties. Take for instance classical thermodynamics, which is not based on the theories of the structure of matter, but is nonetheless conceptually abstract and mathematically complicated. Arnold Sommerfeld (1868–1951), when asked why he had never written a book on thermodynamics (c. 1950), responded humorously:
“Thermodynamics is a funny subject. The first time you go through it, you don't understand it at all. The second time you go through it, you think you understand it, except for one or two small points. The third time you go through it, you know you don't understand it, but by that time you are so used to it, it doesn't bother you anymore.” (commented inAngrist and Helper, 1967, p. 215, also inMasavetas, unpublished manuscript).
Analysis of the organization of physical chemistry in textbooks
As noted in the introduction, there are three principal areas of physical chemistry (PC): (1) equilibrium/thermodynamics, (2) structure/quantum chemistry and statistical thermodynamics, and (3) chemical kinetics or dynamics. In principle these three areas allow for 3! = 6 different sequences. In practice, one usually encounters only four of these sequences: sequence (i): (1) equilibrium, (2) structure, (3) dynamics; sequence (ii): (1) equilibrium, (2) dynamics, (3) structure; sequence (iii): (1) structure, (2) equilibrium, (3) dynamics; sequence (iv): (1) structure, (2) dynamics, (3) equilibrium. Dynamics never appears first in practice, and the sequence structure/dynamics/equilibrium is unusual.Equilibrium deals mainly with phenomenological/classical thermodynamics. It includes usually gases, and solutions of non-electrolytes and electrolytes (the latter accompanied by the study of electrochemistry/electrochemical cells). Structure deals mainly with quantum chemistry, as a rule including or followed by spectroscopy. Structure sometimes includes crystal structure and crystal structure determination by X-ray diffraction. Statistical thermodynamics goes often with (usually after) structure; sometimes it occurs together with, and sometimes it precedes phenomenological thermodynamics. Dynamics deals mainly with chemical kinetics (sometimes including physical kinetics). This includes both phenomenological kinetics and theoretical considerations of kinetics including quantum and statistical treatments.
As was mentioned above, in his book, Moore (#15) considers two alternative approaches to teaching PC. The ‘analytical approach’ treats first matter/substances as we find them in the laboratory, and works its way gradually to the finer states of subdivision. The ‘synthetic approach’ begins with the structure and behavior of matter in its finest known state of subdivision, and progresses gradually from electrons to atoms to molecules, to states of aggregation, and chemical reactions. Sequences (i) and (ii) are consistent with the analytical approach, provided that dynamics/kinetics is split into a phenomenological part that precedes structure and a theoretical/structural part that goes after structure. The analytical approach reflects also the ‘historical development’ of the subject. Sequences (iii) and (iv) are consistent with the synthetic approach.
A considerable number (twenty) of PC textbooks have been surveyed from two perspectives: (i) their contents, organization, and sequencing of the major areas of physical chemistry; (ii) the author's philosophy with respect to the organization and sequencing as indicated by the prefaces or main texts of the books.
Before discussing the analysis of the textbooks, it is very interesting to take a look at a dated but unusually structured book by Cyril Norman Hinshelwood (1897–1967) an English physical chemist, who shared (with the Soviet Nikolay Semenov) the Nobel Prize in chemistry in 1956 for his research into the mechanism of chemical reactions. Hinshelwood considers PC as a difficult and diversified subject. His book titled “The structure of physical chemistry” was published in 1951 and republished as an Oxford Classic Text in the Physical Sciences by Oxford University Press in 2005. The book is divided into six parts as follows: (1) the world as a molecular chaos, (2) control of the chaos by the quantum laws, (3) the electrical basis of matter, (4) forces, (5) the forms of matter in equilibrium, (6) passage towards equilibrium. The main feature is the interplay and connection of structure (quantum chemistry and statistical mechanics) with the phenomenological topics, such as equilibrium. For instance, “The world as a molecular chaos” deals with atoms and molecules, molecular chaos and entropy, thermodynamic principles, aggregates of molecules to solids and liquids, and factors affecting physical and chemical equilibrium, while “Control of the chaos by the quantum laws” covers quantum rules and the absolute position of equilibria. The final part “Passage towards equilibrium” deals with the statistical nature of chemical changes, energy and entropy factors in reaction velocity, some typical reaction mechanisms, propagation of chemical change, and the organic world.
One should also distinguish between theoretical and applied PC, with the former referring mainly to the courses for chemists, physicists and material scientists, and the latter applying to PC courses for chemical engineers, biologists, pharmacists, geologists, etc. Each of these disciplines requires a course with emphasis on different areas. Thus, a course for engineers places the emphasis on thermodynamics, phase-separation processes, and kinetics, while minimizing the coverage of quantum chemistry. For instance, the book “Applied physical chemistry” by Heald and Smith (1974) has chapters on the gaseous state, thermochemistry, equilibrium thermodynamics, phase equilibria, distillation, extraction, crystallization, reaction equilibria, kinetics, electrochemical methods of analysis, and surface chemistry, but does not cover “more academic topics such as the theory of molecular structure”. On the other hand, it contains a chapter on spectroscopic methods of analysis.
Attention will now be focused on the surveyed PC textbooks. As far as it was feasible, the latest or recent editions of the books were consulted. Table 1 lists the reviewed textbooks, and categorizes them according to the sequencing of the various areas of PC.
Categories A–C reflect traditional hierarchies, which place thermodynamics early in the course, adopting the analytical/historic approach. In categories A and B (with four and six books respectively) thermodynamics is preceded by some introductory material (e.g. gases and kinetic theory and sometimes transport properties), while in category C (with three books), thermodynamics is first. Categories A and B differ in the positioning of chemical kinetics and in the treatment of statistical thermodynamics. Category A has kinetics in the traditional order of phenomenological PC: thermodynamics → electrochemistry → chemical kinetics. Category B as well as category C have kinetics after structure (quantum chemistry, statistical thermodynamics, and spectroscopy) in a dynamics/change third part. Also, in category A, books #1 (by Andrews) and #2 (by Adamson) have statistical thermodynamics integrated into thermodynamics, while book #3 (by Metz) has statistical thermodynamics immediately after phenomenological thermodynamic chapters but before electrochemistry, heterogenous equilibria, and solutions. Note that book #9 (by Barrow) in category B also does not have a distinct chapter on statistical thermodynamics but this topic is integrated within (before) thermodynamics.
Categories D and E are represented by just one book each. Book #14 by Levine starts with thermodynamics, has kinetic theory and transport processes before chemical kinetics, and these are followed by quantum chemistry and statistical thermodynamics. Book #15 by Moore has statistical thermodynamics placed early in the course, before quantum chemistry.
Category F (represented by five books) is a very different case. It follows the synthetic approach, having the part on structure (quantum chemistry) first. Book #17 by McQuarrie and Simons (which has the subtitle “A molecular approach”) is unique in that it not only has the chapters on quantum chemistry at the beginning but also systematically integrates the structure of matter into the phenomenological subjects of thermodynamics and chemical kinetics. Similar enhanced treatment of the phenomenological topics is also made by the other four books in this category. Recall that books #1 and 2 of category A have also statistical thermodynamics integrated into thermodynamics. Book #16 (by Pratton, Maron, and Lando) has chapters on gases and liquids and on the solid state before atomic and molecular structure.
The basic features of the hierarchical structure and the contents of the reviewed textbooks are outlined in the Appendix.
Authors' philosophies about textbooks' structure and hierarchy of topics
This section is concluded by focusing on the philosophies of the authors of the various textbooks with respect to the structure and sequencing of the various areas of PC, as indicated by the prefaces of the books. Textbooks that favor the traditional analytical approach will be treated first, and then attention will turn on those favoring the molecular synthetic approach. Many authors, are however, open to alternative approaches where these might be more appropriate for certain instructors and/or students.Moore (#15) starts his text with thermodynamics, “which is based on concepts common to everyday world” (p. 3). Castellan (#10) explicitly labels the topics of quantum chemistry, spectroscopy and statistical thermodynamics as more difficult and considers it advantageous for them to be placed later in the course. Also, he suggests three possible alternative sequences: in the first, he just reverses the order of electrochemistry and kinetics, while in the other two, the above difficult chapters are placed just after the thermodynamics chapters.
Levine (#14) comments that “strictly speaking, the word ‘molecule’ is not part of the vocabulary of thermodynamics” but does not adopt “a purist attitude” and “often uses molecular concepts to help understand thermodynamics” (p. 3); thermodynamics chapters are first and grouped together, followed by electrochemistry, kinetic theory of gases, transport properties and reaction kinetics. Chapters on quantum-chemistry and spectroscopy follow. Statistical mechanics is taken up after thermodynamics and quantum chemistry, and is followed by theories of reaction rates and solids and liquids.
In all editions of his book, Atkins has a central layout, dividing the text into three major parts: (1) equilibrium, (2) structure, (3) change, but he is flexible about the order in which the subject is presented. To this end, Atkins and Aula (#7) provide roadmaps that suggest different course organizations: (a) thermodynamics first, (b) quantum mechanics first. Laidler, Meiser, and Sanctuary (#4) also accept that the sequencing of the various branches is a matter of personal preference.
Switching to the proponents of the molecular approach, it is useful to consider first McQuarrie and Simon's views. The authors start from the tenet that the focus of modern PC is on the molecule, so “by beginning with quantum chemistry, students will learn the fundamental principles upon which all modern physical chemistry is built”.‡ Consequently, “a course in physical chemistry should reflect this viewpoint”, so in their book the authors discuss quantum chemistry first, and then show how it can be applied to a number of model systems. Addressing the students again they state: “Armed with the tools of quantum mechanics, you shall learn that thermodynamics can be formulated in terms of the properties of the atoms and molecules that make up macroscopic chemical systems” (p. xviii).
In the same spirit, Kuhn, Fösterling, and Waldeck (#20) feel that “an early confrontation with quantum mechanics is advantageous to the student” (1st edn, 2000, p. xxv). As a result, they treat first atoms and chemical bonds, and then move to “the more complex representations of matter”. According to the authors, “this organization of the book retains the logical structure of physical chemistry” (p. xxvi). On the other hand, the course can proceed in almost any order. The preface of book #19 by Berry, Rice, and Ross also outlines various sequences that put either quantum mechanics or thermodynamics first. Berry, Rice, and Ross have taught the junior-level PC course both as organized in the text and with the material of part II (statistical mechanics and thermodynamics) preceding that of Part I (The structure of matter).
Authors' views about statistical thermodynamics
Moore (#15) points out that problems studied in chemical thermodynamics and kinetics are ultimately solved by the study of the mechanics of molecules and molecular aggravates, that is, of statistical thermodynamics. For this reason, Moore has placed kinetic theory and statistical thermodynamics early in the text (chapters 4 and 5), just following the thermodynamics chapters. Quantum mechanics and spectroscopy are placed late in the text, but before the solid state, intermolecular forces and the liquid state, and macromolecules.According to McQuarrie and Simon (#17) “statistical thermodynamics provides a way to describe thermodynamics at a molecular level” (p. xviii). In this way, “thermodynamics is simultaneously taught with statistical thermodynamics from a bulk and microscopic viewpoint that enables the student to understand how bulk properties of materials are related to the properties of individual constituent molecules”. Addressing the students, they state:
You shall see that the three laws of thermodynamics can be explained simply and beautifully in molecular terms. We believe that a modern introduction to physical chemistry should, from the outset, develop the field of thermodynamics from a molecular viewpoint. Our treatment of chemical kinetics, which constitutes the last five chapters, develops an understanding of chemical reactions from a molecular viewpoint… quantum chemistry provides the necessary tools to develop a molecular understanding of the rates and the dynamics of chemical reactions” (p. xviii).
Kuhn, Fösterling, and Waldeck (#20) comment that most introductory courses in PC start with the laws of thermodynamics. “When proceeding to classical and then to quantum mechanical statistical thermodynamics it is confusing for the students and difficult for them to accept that the laws of thermodynamics are first set as postulates, then understood as properties of collections of molecules and finally deduced from the postulates of quantum mechanics”. According to the authors, this difficulty is avoided “by first viewing the quantum mechanical nature of matter and then deducing the macroscopic properties as a consequence” (1st edn, 2000, pp. xxvii–xxviii).
Berry, Rice, and Ross (#19) developed the statistical molecular theory and the classical thermodynamics theory “simultaneously … in a mutually reinforcing fashion … (but) if desired the two points of view can be separated” (p. xii). These authors' design of the simultaneous development of classical and statistical thermodynamics aims “to overcome the difficulties associated with the very abstract nature of purely thermodynamic reasoning, and also to illustrate the richness of the phenomena that can arise from molecular interactions” (p. xiii).
Finally, Vemulapalli (#8) maintains that “rigorous treatment of statistical theory before discussing thermodynamics … demands too much from the reader…”. On the other hand, “thermodynamic quantities such as heat capacity and entropy are more interesting when examined from a statistical viewpoint, …”, so “ignoring statistical theory altogether makes classical thermodynamics far less interesting … Hence, one has to weigh the pedagogical benefits against the cost of introducing the submicroscopic theory early”. As a consequence, “a simple heuristic derivation of the Boltzmann distribution law is given in Chapter 2 after a brief discussion of quantum states and microstates” (p. xii).
Conclusions and the way forward
The first research question concerned the connection of the organization/sequencing of the major areas of physical chemistry (PC) in PC textbooks with the logical and psychological structure of PC. It was shown in the rationale of this paper that Jensen's scheme for the logical structure of chemistry leads to a logical structure for PC, and that this structure fixes outer limits, leading to two extreme alternative forms of ‘logic’, and hence two alternative approaches to teaching PC, the ‘analytical approach’, and the ‘synthetic approach’. Further, the discussion has integrated Jensen's scheme and its relationship with the structure of physical chemistry into six categories of hierarchies that were identified from the textbook analysis. As commented already, in Jensen's scheme phenomenological/classical thermodynamics is placed at the molar level of the energy dimension, while quantum chemistry is placed at the electrical level of the structure dimension. In this way, one can place the various PC subjects and topics in the cells of the 3 × 3 Jensen grid. Further examples are shown in Fig. 2. Note that topics such as Born–Haber cycles, ionics and electrodics, reaction mechanisms, and dynamic electrochemistry, which have been placed at the molecular level in Fig. 2, require additional treatment at the electrical level (e.g. electron transfer), but this treatment does not require a mathematical quantum-chemical approach.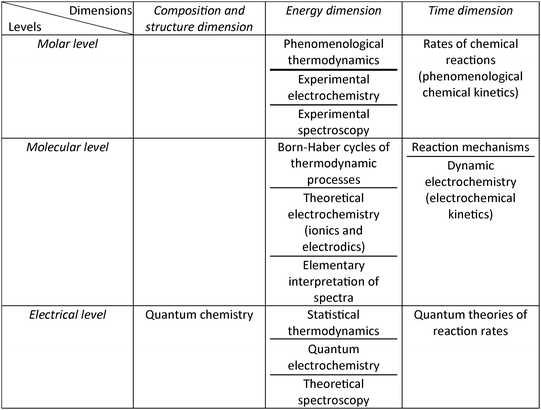 | ||
| Fig. 2 Positioning of various physical chemistry areas and topics in the 3 × 3 Jensen grid, which demonstrates the logical structure of physical chemistry. | ||
Fig. 3 shows various (but not all) possible flows/hierarchies of presenting the various areas of PC. Some examples will be described. The top horizontal arrow shows the analytical approach to PC: phenomenological thermodynamics → electrochemistry → chemical kinetics (category A in our analysis), while the bottom horizontal line is along the submicroscopic/synthetic approach: quantum chemistry → statistical thermodynamics → quantum theories of reaction rates. The latter sequence is usually diverted by turning vertically upwards towards phenomenological thermodynamics at statistical thermodynamics. In this way, kinetics is placed after structure (quantum chemistry, statistical thermodynamics, and spectroscopy) in a dynamics/change part (categories B and C in our analysis). Category D follows the top and bottom horizontal arrows in sequence: phenomenological thermodynamics → chemical kinetics → quantum chemistry → statistical thermodynamics; this sequence can naturally be completed with the quantum theories of reaction rates.
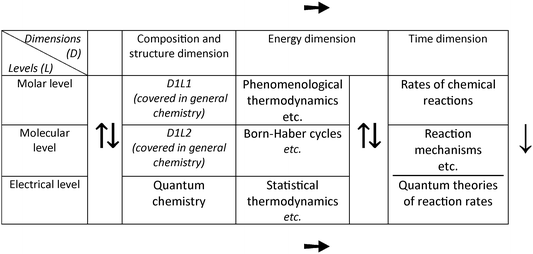 | ||
| Fig. 3 The scheme exhibiting the logical structure of chemistry according to Jensen (1998) and various possible flows/hierarchies of presenting the various areas of physical chemistry. | ||
Fig. 4 derives from Levine (2009, Table 1.1, p. 1) and provides a complementary scheme for the structure of PC; it also shows how the four main areas (thermodynamics, kinetics, quantum chemistry, and statistical mechanics) are related to each other.
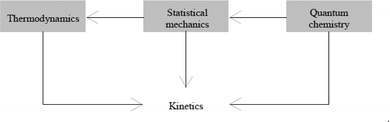 | ||
| Fig. 4 Statistical mechanics serves as the bridge between quantum chemistry and thermodynamics. Kinetics communicates with all of the other areas (Reproduced from Levine (2009) with permission of Mc-Graw-Hill). | ||
It follows from the above that a dichotomy often appears to exist between the macroscopic/phenomenological and the submicroscopic molecular/atomic/electronic approaches to PC. Without implying that the phenomenological subjects are easy – just the opposite, in fact, they are also abstract, and often complex, involving complicated concepts and mathematics – one has to admit that quantum chemistry constitutes the basis of the submicroscopic approach and therefore constitutes a higher learning impediment.
Regarding the second research question about the arguments that support the various textbook organizations, the textbook authors' philosophies with respect to the structure and sequencing of the various areas of PC were explored and found to vary. Most authors favor the traditional analytical approach, while others focus on a molecular synthetic approach, and others who favor an intermediate route. Authors who favor the traditional analytical approach consider the topics of quantum chemistry, spectroscopy and statistical thermodynamics more difficult. On the other hand, the fact that modern PC is based on quantum mechanics makes other authors consider that an early confrontation with quantum mechanics is advantageous to the student. However, many authors are open to alternative approaches. Finally, statistical thermodynamics, despite its conceptual and mathematical complications is judged by many authors essential for developing the phenomenological subjects of thermodynamics and kinetics from a molecular viewpoint, so these authors propose the simultaneous development of classical and statistical thermodynamics. On the other hand, there are authors for whom discussing statistical theory rigorously, before classical thermodynamics, is too demanding for the students, so a simple introduction to the ideas of statistical thermodynamics at the beginning (such as a heuristic derivation of the Boltzmann distribution law) may be preferable.
Final remarks and prospects for further work
As stated already, the ultimate research question of this work is: “Is there an optimum teaching–learning sequence for undergraduate instruction of the various areas of physical chemistry?” The psychological structure of physical chemistry leads to the conclusion that the sequence in which its various areas and topics are presented can be crucial for student understanding. The psychology of learning favors the analytical approach. On the other hand, the synthetic approach may not be favored by the psychology of learning, but has the advantage that it brings students into the realm of modern science (quantum chemistry, spectroscopy, etc.) early in the course, and thus it might catch their interest and motivate them.Of course, the analytical approach is fulfilled in a way by precursor courses such as high school chemistry and general chemistry. These courses include chapters on thermochemistry and thermodynamics, electrochemistry, and chemical kinetics, not to mention the chapters on structure and bonding. The problem with such courses is that they may not be scientifically rigorous, thus promoting many misconceptions. To mention just a few problematic notions: naïve, deterministic pictorial ideas about atomic and molecular orbitals; definition of the orbitals as spaces; the equation of the heat of reaction with the change in enthalpy irrespective of conditions of the system; or the use of concentrations instead of activities in equilibrium constants and in the Nernst equation.
The answer to the ultimate question requires a major project which would attempt, to ‘psychologize’ (Dewey, 1902) the subject matter of physical chemistry, by putting the psychology of learning deeper into the analysis. This can be done by consideration of a wide range of information, for instance input from students and teachers of the courses, consideration of the literature of known student alternative conceptions and frameworks, and how these might influence teaching and learning. Of particular relevance to such a research program are the findings of a qualitative study, in which the present author interviewed chemistry graduate students in order to find out their opinion about the difficulties of the various areas of physical chemistry and their explanations for the difficulties. The findings of this work will be reported in a subsequent publication.
Appendix: basic features of the hierarchical structure and the contents of the reviewed textbooks
Book #1 by Andrews contains a chapter on crystals and liquids after gases. Statistical thermodynamics is integrated into thermodynamics. Chapters on atomic and molecular spectroscopy follow the corresponding chapters on atomic and molecular structure respectively. Concludes with chapters on photochemistry and radiation chemistry, solid state, surface chemistry, macromolecules, entropy and information, bioirreversibility, and biocybernetics.Book #2 by Adamson includes discussion of some additional physical properties after kinetic theory of gases. Statistical thermodynamics is integrated into thermodynamics (as in book #1). Spectroscopy and photochemistry are after quantum chemistry. The book concludes with chapters on solid state, colloids and macromolecules, nuclear chemistry and radiochemistry.
Book #3 by Metz has statistical thermodynamics immediately after thermodynamics. Statistical thermodynamics is followed by electrochemistry, heterogenous equilibria, solutions, and chemical kinetics. Quantum chemistry comes next. Spectroscopy chapters follow the corresponding structure chapters. The book concludes with chapters on intermolecular bonding, crystals, phenomena at interfaces, and macromolecules.
Book #4 by Laidler, Meiser, and Sanctuary follows the traditional route: kinetic theory of gases, thermodynamics, electrochemistry, chemical kinetics, quantum chemistry, spectroscopy, and molecular statistics. The book concludes with chapters on the solid and liquid states, surface chemistry, transport properties, and macromolecules.
Book #5 by Kemp presents physical chemistry in the order: gases and kinetic-molecular theory, thermodynamics, quantum chemistry, spectroscopy, statistical thermodynamics, chemical kinetics, colloids and surface chemistry, polymer chemistry and physics, phase equilibriums, the liquid and solid states.
Book #6 by Winn places kinetic theory of gases after statistical thermodynamics and a chapter on nonequilibrium dynamics after kinetic theory of gases. Chapters on the solid and liquid states are placed after quantum chemistry and before spectroscopy. There are also chapters on thermodynamics of surfaces and interfaces, gravitational, magnetic and electric fields.
Book #7 by Atkins and de Paula has employed, in all editions, a standard layout of the text, dividing it into three parts: (1) equilibrium, (2) structure, (3) change. The book also includes coverage of molecular interactions, soft matter and solids after statistical thermodynamics, but does not include electrochemistry (although previous editions contained three electrochemistry chapters, including a chapter on dynamic electrochemistry).
Book #8 by Vemulapalli has an introductory chapter (molecular statistics) on statistical thermodynamics before thermodynamics, and also includes a standard treatment of statistical thermodynamics at the end. It also includes surface thermodynamics and has spectroscopy chapters among the quantum chemistry chapters. The book presents kinetic theory of gases at the beginning of chemical kinetics and concludes with chapters on optical and electromagnetic properties, the solid state, intermolecular forces, and irreversible processes in the liquid state.
Book #9 by Barrow does not have a distinct chapter on statistical thermodynamics but the topic is integrated within (before) thermodynamics. The book places emphasis on the molecular level, and after discussing the physical properties of gases, moves on to the molecular theory of gases, where apart from classical kinetic theory, an introduction to quantum theory and elementary statistical mechanics are also included. Chapters on thermodynamics and electrochemistry follow, and then come quantum chemistry, spectroscopy, diffraction, electric and magnetic properties, rates and mechanisms, elementary reactions, and finally macromolecular dynamics.
Book #10 by Castellan follows the traditional route: gases and thermodynamics, electrochemistry, chemical kinetics, quantum chemistry, spectroscopy, statistical thermodynamics, and concludes with solids, liquids, surfaces, transport properties, and macromolecules.
Book #11 by Raff discusses the thermodynamics of solids within thermodynamics, and covers the kinetic theory of gases before considering statistical thermodynamics.
Book #12 by Silbey, Alberty, and Bawendi has kinetic theory of gases before chemical kinetics. It concludes with consideration of macromolecules, electric and magnetic properties of molecules, solid state chemistry, and surface dynamics.
Book #13 by Engel and Reid places kinetic theory of gases, transport phenomena, and chemical kinetics at the very end of the book. Spectroscopy chapters occur within the section devoted to quantum chemistry.
Book #14 by Levine treats kinetic theory of gases and transport processes before chemical kinetics, and all these before introducing quantum chemistry. Spectroscopy and photochemistry are discussed after quantum chemistry, and are followed by statistical thermodynamics and theories of reaction rates. The book concludes with consideration of solids, and liquids.
Book #15 by Moore has statistical thermodynamics within thermodynamics, discusses chemical kinetics before electrochemistry and quantum chemistry, and interfaces before quantum chemistry. Spectroscopy and photochemistry are treated after quantum chemistry, and the book concludes with solid state, intermolecular forces, the liquid state, and macromolecules.
Book #16 by Pratton, Maron, and Lando starts with gases and liquids and the solid state, and then goes on to introduce quantum chemistry. This is followed by nuclear chemistry and then by thermodynamics. Statistical thermodynamics is covered after thermodynamics (similar to book #3 by Metz), and is followed by consideration of solutions, electrochemistry, chemical kinetics, and photochemistry. The book concludes with the study of surface phenomena and catalysis, colloids and macromolecules.
Book #17 by McQuarrie and Simons covers quantum chemistry first, including a chapter on computational chemistry. Quantum chemistry is followed by spectroscopy, properties of gases and statistical thermodynamics. Thermodynamics (including solutions and chemical equilibrium) is treated next from a bulk and microscopic viewpoint. The final part of the book includes chapters on kinetic theory of gases, chemical kinetics, gas phase reaction dynamics, and solids and surface chemistry.
Book #18 by Ladd starts with an introductory chapter on structure, energy, and mechanism, before embarking on a long section on the structure of matter (quantum chemistry), which is followed by spectroscopy including X-ray crystallographic analysis. Then come thermodynamics, gases and liquids, and solids. These are followed by solutions, chemical equilibrium and an extensive chapter on electrochemistry. It does not have a distinct chapter on statistical thermodynamics, but relevant topics are included in the chapter on gases and liquids.
Book #19 by Berry, Rice, and Ross has structure of matter (quantum chemistry) as part I, with statistical thermodynamics integrated into thermodynamics. It includes thermodynamics of nonequilibrium processes. Electrochemistry is represented only through solutions of electrolytes. Kinetic theory of gases and kinetic theory of condensed phases precede chemical kinetics. The book concludes with some advanced topics in chemical kinetics.
Book #20 by Kuhn, Fösterling, and Waldeck starts with quantum chemistry, which is followed by spectroscopy. Then follow solids and intermolecular forces, kinetic theory and statistical thermodynamics, thermodynamics, electrochemistry, and chemical kinetics. Chapters on chemical equilibria and reactions are enhanced with a molecular and statistical perspective. It concludes with chapters on macromolecules, organized molecular assemblies, supramolecular machines, and the origin of life (matter carrying information).
Acknowledgements
The author wishes to thank Professor Kyriakos Masavetas of the School of Chemical Engineering of the Technical University of Athens who supplied materials about physical chemistry textbooks from his personal very rich library. Many thanks are also due to the reviewers for their constructive comments that have contributed greatly to the improvement of the manuscript. Finally, the author is grateful to Dr Bill Byers who read the final version and made suggestions for a better presentation.References
- Angrist S. W. and Helper L. G., (1967), Order and Chaos – Laws of Energy and Entropy, New York: Basic Books.
- Armbruster B. B. and Anderson T. H., (1991), Textbook analysis, curriculum components, and conceptual framework, in Lewy A. (ed.), The International Encyclopedia of Curriculum, Oxford: Pergamon Press, pp. 78–81.
- Ball D. L. and Cohen D. K., (1996), Reform by the book: what is: or might be: the role of curriculum materials in teacher learning and instructional reform? Educ. Res., 25(6), 8–14.
- Birkoff G. and von Newmann J., (1936), The logic of quantum mechanics, Ann. Math., 37, 835–843.
- Britton B. K., Woodward A. and Binkley M., (ed.), (1993), Learning from textbooks theory and practice, Hillsdale, New Jersey Hove and London: Lawrence Erlbaum Associates.
- Castro E. A. and Fernandez F. M., (1987), Intellectual development beyond formal operations, Int. J. Sci. Educ., 9, 441–447.
- Chiappetta E. L. and Fillman D. A., (2007), Analysis of five high school biology textbooks used in the United States for inclusion of the nature of science, Int. J. Sci. Educ., 29, 1847–1868.
- Davis E. A. and Krajcik J., (2005), Designing educative curriculum materials to promote teacher learning, Educ. Res., 34(3), 3–14.
- Derrick M. E. and Derrick F. W., (2002), Predictors of success in physical chemistry, J. Chem. Educ., 79, 1013–1016.
- Dewey J., (1902), The essential Dewey, Pragmatism, education, democracy, Chicago, IL: University of Chicago Press, vol. I.
- Dirac P., (1929), Quantum mechanics of many-electron systems, Proc. R. Soc. London Ser. A, 123, 714–733.
- diSessa A. A., (1993), Toward an epistemology of physics, Cogn. Instr., 10(2 & 3), 105–225.
- Flamm D., (1997), History and outlook of statistical physics, Report number UWThPh-1997-52: http://arXiv:physics/9803005v1 [physics.hist-ph], http://arxiv.org/pdf/physics/9803005v1.pdf, accessed on 10 March 2014.
- Friedmann H. C., (1990), Fifty-sic laws of good teaching, J. Chem. Educ., 67, 413–414.
- Getman F. H., (1913), Outlines of theoretical chemistry, New York: Wiley.
- Glasstone S., (1940), Textbook of physical chemistry, New York: Van Nostrand.
- Hahn K. E. and Polik W. F., (2004), Factors influencing success in physical chemistry, J. Chem. Educ., 81, 567–572.
- Heald C. and Smith A. C. K., (1974), Applied physical chemistry, London and Basingstoke: MacMillan.
- Herron J. D., (1978), Piaget in the classroom, J. Chem. Educ., 55, 165–170.
- Jensen W. B., (1998), Logic, history, and the chemistry textbook, I: Does chemistry have a logical structure? J. Chem. Educ., 7, 679–687.
- Johnstone A. H., (1991), Thinking about thinking, Int. Newsl. Chem. Educ., 6, 7–11.
- Johnstone A. H., (2000), Teaching of chemistry – logical or psychological?, Chem. Educ. Res. Pract., 1, 9–15.
- Kahveci A., (2010), Quantitative analysis of science and chemistry textbooks for indicators of reform: a complementary perspective, Int. J. Sci. Educ., 32, 1495–1519.
- Lagowski J. J., (ed.), (2004), Chemistry – Foundations and applications, Farmington Hills, MI: MacMillan Reference, vol. 1–3.
- Levine I. N., (2009), Physical chemistry, 6th edn, New York: Mc-Graw-Hill.
- Masavetas A. K., Mathematical improprieties in dealing with the criteria for thermodynamic spontaneity and thermodynamic equilibrium in physicochemical changes (in Greek), Athens, Greece: National Technical University of Athens, unpublished manuscript.
- McQuarrie D. A. and Simon J. D., (1997), Physical chemistry – a molecular approach, Saulito California: University Science Books.
- Moore R. J. and Schwenz R. W., (1992), The problem with P. Chem., J. Chem. Educ., 69, 1001–1002.
- Nicoll G. and Francisco J. S., (2001), An investigation of the factors influencing student performance in physical chemistry, J. Chem. Educ., 78, 99–102.
- Novak J. D., (1990), Concept mapping: a useful tool for science education, J. Res. Sci. Teach., 27, 937–949.
- Pauling L. and Wilson E. B. Jr., (1935), Introduction to quantum mechanics with applications to chemistry, New York: McGraw-Hill.
- Pilar F. L., (1968), Elementary quantum chemistry, New York: McGraw-Hill.
- Sözbilir M., (2004), What makes physical chemistry difficult? Perceptions of Turkish chemistry undergraduates and lecturers, J. Chem. Educ., 81, 573–578.
- Sutton M., (2003), The father of physical chemistry, Chemistry World, Issue 5, May 2003, http://www.rsc.org/chemistryworld/Issues/2003/May/physicalchem.asp, accessed on 10 March 2014.
- Taber K. S., (2013), A common core to chemical conceptions: learners' conceptions of chemical stability, change and bonding, in Tsaparlis G. and Sevian H. (ed.), Concepts of matter in science education, Dordrecht: Springer, pp. 391–418.
- Tsaparlis G., (1997), Atomic and molecular structure in chemical education – a critical analysis from various perspectives of science education, J. Chem. Educ., 74, 922–925.
- Tsaparlis G., (2008), Teaching and learning physical chemistry – review of educational research, in Ellison M. D. and Schoolcraft T. A. (ed.), Advances in teaching physical chemistry, Washington, DC: American Chemical Society/Oxford University Press, pp. 75–112.
- Tsaparlis G. and Sevian H., (2013). Introduction: Concepts of matter – complex to teach and difficult to learn, in Tsaparlis G. and Sevian H. (ed.), Concepts of matter in science education, Dordrecht: Springer, pp. 1–8.
- Weiss I., Pasley J., Smith P., Banilower E. and Heck H., (2003), Looking inside the classroom: a study of K-12 mathematics and science education in the United States, Chapel Hill, NC: Horizon Research.
Footnotes |
| † This is truer in the secondary level (and especially the upper secondary). At the tertiary level, the dependence of instructors on the textbook varies, but as a rule, they provide students with lists of recommended textbooks, which they follow to a varying extent in their teaching. The dependence of the instructors and of the students on the textbooks varies across various countries: in some cases the textbook(s) is(are) the main means of study for the students (who often also consult their class-notes), while in other cases, the textbook(s) is/are supplementary to what the instructors teach. Relevant here is a list of fifty-six rules of good teaching, in which the very first one reads: “Choose a good textbook, but do not follow it in lectures” (Friedmann, 1990, p. 413). Needless to add that the dependence on the textbook(s) also varies within the same country and even the same institution, depending on the instructor. In any case, it must be accepted that textbooks inevitably play a leading role in shaping instructors’ views and philosophies about the teaching content and its sequencing. |
| ‡ The importance of quantum mechanics for modern applications is also emphasized by authors who place thermodynamics first. For instance, Engel and Reids (#13) present exciting new applications of quantum behavior, such as band-gap engineering, quantum dots, quantum wells, teleportation, and quantum computing. |
| This journal is © The Royal Society of Chemistry 2014 |
