Rethinking chemistry: a learning progression on chemical thinking
Hannah
Sevian
a and
Vicente
Talanquer
b
aDepartment of Chemistry, University of Massachusetts Boston, Boston, MA 02125, USA. E-mail: hannah.sevian@umb.edu
bDepartment of Chemistry and Biochemistry, University of Arizona, Tucson, Arizona 85721, USA. E-mail: vicente@u.arizona.edu
First published on 26th September 2013
Abstract
Dominant educational approaches in chemistry focus on the learning of somewhat isolated concepts and ideas about chemical substances and reactions. Reform efforts often seek to engage students in the generation of knowledge through the investigation of chemical phenomena, with emphasis on the development and application of models to build causal explanations and predict outcomes. However, chemistry has been characterized as a technoscience that blends scientific pursuit and technological goals. Besides searching for explanations, our discipline also involves the design of substances and processes to address relevant problems, as well as the evaluation of social, economic, and environmental benefits, costs, and risks associated with chemical knowledge and products. In order to develop authentic curricula, instruction, and assessments that are better aligned with the core goals and practices of chemistry, we need to understand how students' chemical thinking progresses over time. We define chemical thinking as the development and application of chemical knowledge and practices with the main intent of analyzing, synthesizing, and transforming matter for practical purposes. In this paper we present a blueprint of a theoretically sound and evidence-based foundation for an educational framework centered on the idea of chemical thinking. Our investigations are focused on the development of a learning progression that describes likely pathways in the evolution of students' chemical thinking with training in the discipline from grade 8 (age 13–14) through 16 (undergraduate completion).
Introduction
Dominant approaches to the teaching of chemistry in many countries tend to present the discipline as a collection of somewhat isolated topics: atomic structure, chemical reactions, chemical bonding, thermodynamics, kinetics, etc. Chemical ideas and practices are also often introduced devoid of a clear practical purpose, beyond that of explaining the properties of matter (Van Berkel et al., 2000; Eilks et al., 2013). This approach to teaching chemistry is disconnected from the major purposes of the chemical enterprise; it fails to engage students in learning how to pose and answer questions that reflect authentic chemical concerns: how do we identify, avoid, or mitigate the effects of pollutants in our environment? How do we synthesize new medicines? The topic-centered conceptualization of school chemistry stands in stark contrast to current visions for science education in the US (NRC, 2011), as well as in other countries (Waddington et al., 2007; Osborne and Dillon, 2008), in which students are expected to “… over multiple years of school, actively engage in science and engineering practices and apply crosscutting concepts to deepen their understanding of each field's disciplinary core ideas” (NRC, 2011, p. 2).A central challenge in science education is the development of coherent educational models that integrate central ideas and practices within and across disciplines. One possible approach to facing this challenge is through the development of curricula and instructional models that actively and meaningfully engage students in authentic domain-specific practices, meaning the specific ways of reasoning, doing, and valuing that characterize each scientific domain (Bulte et al., 2006; Talanquer, 2013a). In the case of chemistry, those authentic practices certainly involve the investigation of chemical substances and phenomena in the search for explanations for their properties and behaviors. However, chemistry has been characterized as a technoscience that blends the pursuit of scientific knowledge with technological goals driven by human needs and conditions (Bensaude-Vincent and Simon, 2008; Chamizo, 2013). As such, chemical scientists also engage in practices that are akin to those of engineers, involving the design, application, and evaluation of methods and strategies to analyze, synthesize, and transform chemical substances (NRC, 2003).
The concept of chemical thinking can be used to capture the knowledge, reasoning, and practices that characterize the chemical enterprise (Talanquer and Pollard, 2010). We define chemical thinking as the development and application of chemical knowledge and practices with the main intent of analyzing, synthesizing, and transforming matter for practical purposes. The core ideas and theoretical and experimental practices used to identify substances, synthesize new materials, and transform matter uniquely define chemistry as a discipline. They have also had a profound impact on modern societies. They have transformed the way we live and have helped increase the quality of human life. They have also opened the door to serious environmental and social problems. Understanding the ideas and practices on which chemical thinking is based is therefore critical for individuals who will rely on their chemical understanding to make important decisions in the practice of their profession. The foundations of this understanding are also critical for scientifically literate individuals who will not necessarily pursue career pathways that involve the discipline of chemistry. Throughout their lives, these individuals will have to make important decisions that rely on fundamental chemical thinking to answer questions such as how to dispose appropriately of material waste, what containers are safe for storing different foods, and which fuels impart the least damage to the planet.
Common teaching approaches in chemistry tend to focus on engaging students in the description of the structure of chemical substances and the characteristics of chemical processes (Van Berkel et al., 2000). Recent educational reform efforts seek to shift teaching practices to actively involve students in authentic investigations that create opportunities for modeling and sense-making (NRC, 2011). From our perspective, some of these efforts tend to overemphasize the investigative and explanatory aspects of chemistry at the expense of other core practices (Talanquer, 2013a). A more authentic balance needs to be achieved by also opening spaces for students to engage in the design of materials or processes, and in the evaluation of the benefits, costs and risks associated with the use and transformation of chemical entities. Integrating these other aspects of chemistry would help us foster students' abilities to make informed decisions, build justifications, evaluate outcomes, and test ideas in the context of personal, societal, and global concerns.
In recent years, chemistry educators across the world have attempted to transform chemistry education based on the idea of context-based learning (Bulte et al., 2006; Schwartz, 2006; Eilks et al., 2013). The associated curricula use everyday contexts and applications of chemistry as starting points for developing student understanding. Several of these approaches engage students in authentic practices and seek to connect key ideas and skills in meaningful ways (King, 2012). Nevertheless, in some cases core chemistry ideas and practices get buried under the specific contexts used to motivate and drive learning in the classroom. When this happens, learning about the particular problem under analysis (e.g., global warming) and the specific chemistry concepts related to it (e.g., properties of greenhouse gases) become the central focus of attention, rather than the analysis, discussion, and reflection of the core chemistry ideas, ways of thinking, and experimental strategies that can be applied to understand and solve such types of problems.
Given the above ideas and concerns, our work has focused on building a theoretically sound and evidence-based foundation for a coherent educational framework centered on the idea of chemical thinking, which can guide the transformation of curriculum, instruction and assessment in chemistry. Our work is being informed by careful analyses of the nature of our discipline (Talanquer, 2013a), the relevance of chemical thinking to modern citizenry (Sjöström, 2013), and by research on how students learn chemistry over time. In this paper we present a blueprint of this foundation, together with a description of our research efforts to actually build it. Our investigations are focused on the development of a learning progression (LP) that describes likely pathways in the evolution of students' chemical thinking with training in the discipline from grade 8 (age 13–14) through 16 (undergraduate completion).
Theoretical framework
Learning progressions
In the past few years there has been a surge of interest in the development of learning progressions (LPs) of central ideas in the sciences that can serve as curriculum models and assessment frameworks in educational settings. These LPs describe successively more sophisticated ways of thinking about a topic (NRC, 2007; Corcoran et al., 2009) and are based on educational research about how people learn, existing pedagogical content knowledge in the area of interest, as well as on the critical analysis of the structure of the associated disciplinary knowledge. To date, educational researchers have developed LPs in science for diverse topics such as atomic–molecular structure (Smith et al., 2006; Stevens et al., 2010), force and motion (Alonzo and Steedle, 2009), genetics (Duncan et al., 2009), the theory of evolution (Lehrer and Schauble, 2012), scientific argumentation (Berland and McNeill, 2010), and energy (Lacy et al., 2012). However, there is still ample debate on issues such as what constitutes progress in a given area, how more sophisticated ways of thinking are characterized, and whether progress can be described as a series of successive levels of understanding (Sikorski and Hammer, 2010). Debate also exists on whether intermediate levels in a progression should only include simplified ideas that are scientifically sound, or whether describing scientifically inaccurate intermediate understandings may also be productive (Duncan and Rivet, 2013).The promise of LPs lies in the potential to guide the coordination of teaching, instructional resources, and assessment with cognitive and metacognitive practices so that learning builds coherently. However, the field has not yet come to consensus on a more precise definition of what an LP is. Recently, Duschl and collaborators (Duschl et al., 2011) conducted a comprehensive review of LP research in science education across the US and Europe over the past decades. These authors reviewed critical foundational domains that have contributed to the development of LP research, including work in the areas of conceptual change, didaktik and teaching experiments in the European tradition of education (e.g., Model of Educational Reconstruction), and learning trajectories in mathematics education.
In their review, Duschl and collaborators focused on describing how LPs are being created, and how they are being validated and described. They isolated four major aspects in which existing LPs tend to vary: (1) their focus, as some LPs center on scientific knowledge without integrating science practices, while others focus on science practices without integrating domain knowledge; (2) the nature of their boundaries, as the definition of lower and upper levels (also called lower and upper anchors, respectively) varies across LPs; (3) the characteristics of intermediate states, as the manner in which different levels of understanding are studied, described, and related to instruction ranges from linear sequences of steps to complex networks of ideas, with some LPs mostly independent of instruction and others tightly linked to it, and (4) the associated models of conceptual change, as some LPs are based on a misconception-based ‘fix it’ view, while others take an evolutionary ‘work with it’ perspective.
Although the current number of investigations on LPs about different topics is relatively small, there are important studies directly or indirectly focused on core ideas in chemistry. Such is the case with the LPs associated with atomic–molecular structure (Smith et al., 2006; Stevens et al., 2010), properties of matter (Smith et al., 1985, 2010; Liu and Lesniak, 2005), the concept of substance (Johnson and Tymms, 2011), and carbon cycling (Mohan et al., 2009). Many of the findings described in these studies provide foundational ideas for initial hypotheses for our own chemical thinking learning progression (CTLP). Assessment work, such as that of Claesgens et al. (2009) on changes in student reasoning about matter, energy, and change, also provides insights into how chemistry students' ideas and ways of reasoning may evolve over time. Before we describe our approach to the development and validation of the CTLP, which is a blended research pursuit and educational development effort, it is important to lay out the fundamental theoretical commitments that form the foundation under this work.
Theoretical commitments
Various chemists and philosophers of science have argued that chemical scientists engage in well-defined approaches to practicing chemistry. Breslow defines the work of chemists as consisting of two different types of activity: “investigat[ing] the natural world and try[ing] to understand it, while other chemists create new substances and new ways to perform chemical changes that do not occur in nature” (Breslow, 1997, p. 2). Others emphasize that, beyond increasing knowledge, chemists are obligated to engage in evaluative activity as well (Vilches and Gil-Pérez, 2013). In our view, the teaching of chemistry should rely on three major pedagogical approaches that reflect core activities in our discipline: investigation, design, and evaluation. Investigation refers to inquiry practices focused on the description, understanding, and prediction of chemical properties and phenomena. These types of activities involve the design and implementation of investigations to answer relevant questions about systems of interests, as well as the development and application of models to make sense of observations, build explanations, or predict outcomes. Major curricular and reform efforts in both school (K-12) and university chemistry have been largely based on this type of pedagogical approach. For example, the science process skills movement in the 1980s (Padilla et al., 1983; Padilla, 1986), the inquiry emphasis in the 1990s and 2000s (NRC, 1996), the 5E and 7E instructional design models (Bybee, 1997; Eisenkraft, 2003; Bybee et al., 2006), and most recently, the incorporation of science practices in the framework for K-12 Science Education in the US (NRC, 2011).
The process of design in chemistry involves the creation and implementation of strategies for analyzing, synthesizing, or transforming substances to address relevant problems. Engineering design (French, 1999) and technology design (Dugger, 2001) have some overlap with how chemists engage in these types of processes. Currently, a design-based pedagogy is largely absent from school chemistry in the US, but it becomes increasingly important as students advance in their chemistry training at the university level. In an educational setting, design activities should engage students in brainstorming ideas, identifying and analyzing constraints, weighing the benefits and tradeoffs of alternative plans, making decisions, testing ideas, and evaluating outcomes. Many of these aspects of engineering design have been included in the Framework for K-12 Science Education (NRC, 2011), and incorporated into the Next Generation Science Standards (NRC, 2013). Thus, as new standards become adopted in the US, there may be an increasing emphasis on a design-based pedagogy in school chemistry.
The third type of authentic activities, labeled evaluation, is concerned with considering, weighing, and judging the social, economic, and environmental benefits, costs, and risks of chemical products and activities. The development and use of chemical substances and processes demand the consideration of moral and ethical issues. In an educational setting, evaluation activities should thus engage students in analyzing complex and controversial problems or dilemmas, connecting ideas, building arguments, and making decisions while taking into consideration social, economic, political, and ethical factors. An evaluation-based pedagogy is also largely absent from school science in the US, though is incorporated in such efforts as Science, Technology, and Society initiatives (Bybee, 1987; Solomon, 1996; Eilks et al., 2013), and Socio-Scientific Issues perspectives (Zeidler et al., 2005). Its presence in university chemistry education ranges from absent to considered to some extent, depending on factors such as geography, values and research areas of faculty, mission of the chemistry department or higher education institution, and national political interests. For example, some chemistry departments include expertise in green chemistry, which emphasizes this pedagogical approach to chemistry research.
The development and validation of the CTLP described in this paper is ultimately concerned with characterizing learning pathways through which students' ideas and ways of thinking about synthesis, analysis, and transformation (i.e., their chemical thinking) develop through formal education, in the context of pedagogical approaches that involve investigation, design, and evaluation. This approach to the conceptualization of chemistry education shares common features with models of competence used to develop chemistry education standards in some European countries (Waddington et al., 2007). For example, “investigation” and “evaluation” are areas of expected competence in German science education standards (Schecker and Parchmann, 2007).
| Crosscutting disciplinary concept | Core question |
|---|---|
| Chemical identity | How do we identify chemical substances? |
| Structure–property relationships | How do we predict the properties of materials? |
| Chemical causality | Why do chemical processes occur? |
| Chemical mechanism | How do chemical processes occur? |
| Chemical control | How can we control chemical processes? |
| Benefits–costs–risks | How do we evaluate the impacts of chemically transforming matter? |
Chemical identity. The development of analytical techniques to identify and detect chemical substances in our surroundings, as well as strategies to synthesize chemical compounds, is based on the central assumption that each chemical substance has a differentiating property that makes it unique (Enke, 2001). However, understanding which properties may be used as differentiating characteristics for chemical substances is not straightforward. Relevant research suggests that novice learners' ideas and decisions about identity and category membership are constrained by the surface features and appearances of the systems of interest (Vosniadou and Ortony, 1989; Talanquer, 2009). Additionally, students tend to identify a sample of material by its history (origin and what has happened to it) rather than physical and chemical properties (Johnson, 2000). Also, they often fail to understand the difference between substance and object (Au, 1994), and between extensive (e.g., mass) and intensive (e.g., density) properties (Wiser and Smith, 2008).
Structure–property relationships. Making decisions about what type of substance to synthesize, what reactant to use, or what detection technique to utilize critically depends on the understanding of the relationship between properties and structure at different scales. Many physical and chemical properties emerge from the dynamic movement and interactions between the myriads of particles that make up a macroscopic sample of a substance (Chi, 2005; Levy and Wilensky, 2009). However, research indicates that novice learners tend to rely on an additive versus an emergent framework in the prediction of the physical and chemical properties of substances (Talanquer, 2008). Students implicitly presuppose that substances directly inherit properties from their individual submicroscopic components (Talanquer, 2006; Taber and García-Franco, 2010). In general, students have difficulties reasoning about systems and processes at multiple scales, focusing on surface features of a system to build predictions and make decisions (Gilbert and Treagust, 2009; Cooper et al., 2012, 2013).
Chemical causality. The atoms, ions, or molecules that make up a chemical substance adopt stable dynamic structures determined by the nature of random interactions between particles in the system. Stable structures may undergo transformations via random interactions with other particles. The likelihood of such transformations depends on both the stability of the new structures and the probability of successful interactions between particles in the system (Atkins and de Paula, 2006). Educational research suggests that novice learners rarely adopt a causal framework in which structures and events result from dynamic random interactions among multiple components. On the contrary, many students assume that phenomena are induced by leading agents acting on passive entities (Andersson, 1986; Grotzer, 2003), or consider that processes are driven by an agent's desire or purpose (Taber, 2009, 2013; Talanquer, 2010). These ways of thinking constrain students' ability to analyze chemical phenomena which frequently involve simultaneous random processes that occur with different probabilities.
Chemical mechanism. The prediction and control of the outcome of a chemical process depends on the understanding of the sequence of events triggered by the combination of chemical reactants. The identification of reaction mechanisms relies on theoretical models about the structure of matter that can be used to explain and predict properties and behaviors (Carroll, 1998). Relevant educational research indicates that many novice learners think of chemical reactions as mixing or relocation processes, with no clear sense of what happens during a chemical change (Andersson, 1990; Hesse and Anderson, 1992). In general, they have a tendency to describe the evolution of a chemical process as a linear chain of events (story or chronology). Each variable is considered one at a time, assigning a preferred direction to the process (Talanquer, 2006). Sequence of events in chemical reactions are built based on surface structural features rather than on the analysis of chemical properties and interactions (Bhattacharyya and Bodner, 2005).
Chemical control. The design of successful strategies to identify or synthesize a chemical substance depends on the understanding of internal and external factors that affect the stability and reactivity of chemical substances. It is also critical to understand how such factors affect the rate of chemical processes and the extent to which these processes can go to completion (NRC, 2003). Research in this area suggests that many novice learners implicitly assume that chemical processes always need to be initiated by active agents and that, once started, they always go to completion (Johnson, 2000, 2002; Taber and García-Franco, 2010). In general, students struggle to differentiate those factors that affect the rate of a chemical process versus those that determine the final state of equilibrium (Gilbert et al., 2002). In general, one can expect novice learners to conceive chemical control as something achieved by modifying external parameters, such as temperature, neglecting internal factors such as the structure of the particles involved (Kind, 2004).
Benefits–costs–risks. The analysis, synthesis, and transformation of chemical substances have many benefits for modern societies. However, there are also social, political, economic, and environmental costs and risks that need to be taken into account when making decisions involving the application of chemical design (Bensaude-Vincent and Simon, 2008). Research in the area of argumentation of socio-scientific issues suggest that individuals tend to selectively credit or dismiss evidence of benefits, costs, and risks based on personal values that they share with others rather than on scientific knowledge (Kahan et al., 2011). In the context of science education, science learners have been found to rely on emotive, intuitive, and rationalistic resources when analyzing socio-scientific issues, independently of their level of content knowledge about a subject (Sadler and Donnelly, 2006). Changes in students' ability to generate high-quality costs–benefits and risk analyses seem to vary in a non-linear fashion with content knowledge acquisition (Sadler and Fowler, 2006).
Stepping stones represent productive ways of thinking that may support important reconceptualizations with proper instruction. For example, the analysis of children's ideas about material kinds (chemical identity) reveals that at young ages materials are thought of as objects that can be categorized into different classes based on perceptual features (Wiser and Smith, 2008). It is not until children start thinking of materials as basic “constituents” of matter that they can begin to develop an understanding of the concept of substance (Johnson, 2000). Thus, conceptualizing materials as fundamental constituents rather than as simple labels used to describe different types of “stuff” can be seen as an stepping stone in the path to meaningfully understand what a chemical substance is and what cues are most relevant in assigning chemical identity.
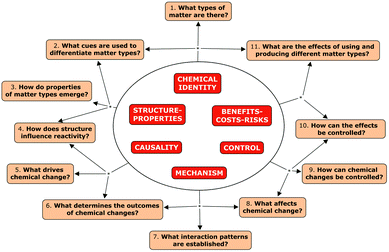
The progress variables in our CTLP have been selected to track students' conceptual sophistication and modes of reasoning about the six crosscutting disciplinary concepts described in the previous section. Conceptual sophistication is determined by the nature of students' underlying assumptions about the structure and properties of chemical entities and phenomena. Modes of reasoning refer to the complexity of student reasoning in terms of their ability to connect ideas, build justifications, make decisions, and construct sophisticated explanations. To facilitate the characterization of assumptions and modes of reasoning we have identified a set of essential questions that define the critical aspects of students' chemical thinking that we propose to explore (see Fig. 2). These essential questions are fundamental queries in the work of chemical scientists as they analyze materials, synthesize new substances, and transform matter. The answers given to these questions can be expected to depend on people's conceptual sophistication and modes of reasoning as related to the six crosscutting disciplinary concepts represented in Fig. 2. These essential questions define the progress variables along which chemical thinking is hypothesized to develop with training in the discipline.
A variety of researchers interested in conceptual change have identified diverse cognitive elements that seem to guide, support, but also constrain students' reasoning in different domains. They have referred to these cognitive elements in different ways, such as implicit presuppositions (Vosniadou, 1994), phenomenological primitives (diSessa, 1993), ontological beliefs (Chi, 2008), conceptual resources (Redish, 2004), and core knowledge (Spelke and Kinzler, 2007). Nevertheless, there is debate on the extent to which these cognitive elements are better described as coherent integrated knowledge systems versus fragmented collections of cognitive resources (Brown and Hammer, 2008; diSessa, 2008; Vosniadou et al., 2008). It is likely that their level of integration may vary depending on the nature of the knowledge domain and the prior knowledge and experiences of each individual.
Based on these ideas, our research has been based on a theoretical model that proposes that student reasoning in chemistry is often guided by implicit or explicit assumptions about the nature of chemical entities and processes (Talanquer, 2006, 2009; Maeyer and Talanquer, 2013). The nature of these cognitive elements may change over time with development and learning; some of these constraints may lose or gain strength depending on existing knowledge, contextual features, and perceived salient cues and goals of a task. Nevertheless, these assumptions support the development and application of dynamic mental models of the systems of interest, and help us make decisions about what behaviors are possible or not and about what variables are most relevant in determining behavior. They also support the development or application of reasoning strategies to make predictions about how the object will behave when involved in different processes or events. Thus, the characterization of progress in understanding may be facilitated by mapping the landscape of assumptions that most commonly guide student reasoning when engaged in disciplinary tasks. Talanquer (2009) has illustrated this approach in the analysis of students' evolving ideas about the particulate model of matter. For example, many learning difficulties in this area seem to be associated with learners' tendency to assume that substances are homogeneous entities, and thus their properties are the same at all scales (e.g., atoms and molecules have the same density or melting point as the macroscopic sample of which they are part).
| Mode | Description |
|---|---|
| Descriptive | Salient entities in a system are identified or recognized. Explicit properties are described, verbalized. Functions or properties of entities are seen as sufficient explanation for their behavior. A phenomenon is seen as an instantiation of reality; it may be re-described by merely asserting that things are as they are without referring to causes. Reasoning mostly based on experiences and knowledge from daily life. Strong influence of surface similarity and recognition on judgment and decision making. |
| Relational | Salient entities in a system are identified or recognized. Explicit and implicit differentiating properties are highlighted. Spatial or temporal relations between entities are noticed. Correlations between properties and behaviors are established but not explained or justified. A phenomenon is seen as an effect of a single entity, the natural outcome of a single property or the linear combination of several properties; no mechanisms are proposed. Reduction of variables and overgeneralization constrain reasoning. |
| Linear causal | Salient entities in a system are identified or recognized. Explicit and implicit differentiating properties are highlighted. Spatial or temporal organization of and connections between entities are noticed. Relevant direct interactions between entities invoked. Although the influence of many factors may be recognized, phenomena tend to be seen as (reduced to) the result of the actions of a single agent on other entities; proposed mechanisms involve linear cause–effect relations and sequential chains of events. Reduction of variables and overgeneralization frequently constrain reasoning. |
| Multicomponent | Salient entities in a system are identified or recognized. Explicit and implicit differentiating properties are highlighted. Spatial or temporal organization of and connections between entities are noticed. Relevant interactions between entities are invoked. Effects of several variables are considered and weighed. |
| (a) Isolated | (a) Complex phenomena are seen as the result of the static or dynamic interplay of more than one factor and the direct interactions of several components. Effects of several variables are considered and weighed separately. |
| (b) Integrated | (b) Complex phenomena are seen as the result of the dynamic interplay of more than one factor and the direct and indirect interactions of several components. Explanations as interconnected stories of how different variables affect the entities involved. The effects of different variables are more thoroughly and systematically explained for multiple entities involved. |
Development and empirical validation
Our research work in the development and validation of the CTLP is guided by the following overarching research questions:(1) What core assumptions and modes of reasoning can be expected to characterize novice (lower anchor) and sophisticated (upper anchor) chemical thinking as related to each of the six crosscutting disciplinary concepts: chemical identity, structure–property relationships, chemical causality, mechanism, chemical control, and benefits–costs–risks?
(2) What core assumptions and modes of reasoning may constrain students' thinking about each of the six crosscutting disciplinary concepts at intermediate learning stages?
(3) What ‘stepping stones’ characterize learning pathways in the progression from novice to sophisticated chemical thinking?
To illustrate our approach to developing and validating the CTLP, we briefly outline examples of the application of our framework to the analysis of data collected using an instrument (GoKart interview, see Table 3) designed to explore students' conceptual sophistication and modes of reasoning as related to chemical identity, structure–property relationships, and benefits–costs–risks. The GoKart interview protocol is being used by teachers in middle and high schools in an urban school district in the Northeast, and by researchers at two universities in the US and one in Costa Rica. The instrument was designed to uncover students' assumptions and modes of reasoning when engaged in an evaluation task that requires the selection of the most appropriate fuel to use in the design of a GoKart. The task thus demands students to apply chemical thinking in the areas of chemical analysis and transformation. In the context of this problem, participants are asked a series of questions that probe their reasoning about the use of different fuels based on information about their physical properties, chemical composition, and molecular structure. An important feature of the GoKart instrument is that there is no one right answer to the choice of fuel. The final decision depends on the relative weight that people assign to different factors when making a decision. Even experts do not agree on the best answer.
| Interview question | Question intent | |
|---|---|---|
| 1 | Which fuel would you use? Why? (list of fuels and each one's main chemical component is provided) | • Generate mental model of the scenario |
| • Determine immediately accessible prior knowledge about the fuels and bases for decision-making | ||
| • Determine participant's thinking on whether and how octane from petroleum vs. from wood pellets differ | ||
| 2 | Gasoline and E85 are liquids, while natural gas is available as a gas. Is this important? Why? | • Determine how the participant considers state of matter |
| • Determine participant's thinking on how state of matter influences fuel usage, reactivity, outcomes, and consequences of use | ||
| 3 | E85 contains carbon, hydrogen and oxygen, while the other two fuels contain only carbon and hydrogen. Is this important? Why? | • Determine how the participant considers composition of matter |
| • Determine participant's thinking about how composition influences properties, outcomes, and consequences of use | ||
| 4 | Are the molecular structures of the fuels important? Why? (ball-and-stick drawings, with element symbols added, are provided) | • Determine how the participant considers molecular structure |
| • Determine participant's thinking about how molecular size, shape, and bonding/connectivity influence properties, outcomes, and consequences of use | ||
| 5 | In terms of how the fuels affect the environment, is one fuel better than the others? Why? | • Determine what economic, environmental, social, political, ethical, and moral factors the participant views as important to consider in decision-making |
| • Assess how participant evaluates benefits and costs associated with the use of different fuels | ||
We have interviewed individuals ranging from students in grade 8 (age 13) through graduate school (e.g., fifth-year PhD chemistry students), and chemistry experts in academia, industry, and government/regulatory work. The examples that are analyzed below are representative of the full data set, and illustrate different levels of conceptual sophistication and the application of various modes of reasoning along progress variable 2 in Fig. 2 (What cues are used to differentiate matter types?).
Let us first consider the case of a tenth grader (code name Maria) taking an introductory chemistry course in high school who was interviewed by her teacher. As illustrated by the following excerpt, her reasoning was strongly influenced by the assumption that natural substances were somehow better for the environment than fuels she perceived as not coming from nature:
Interviewer : so if all the fuels cost the same per gallon, which fuel would you choose to power your GoKart and why?
Maria : well, I wanna consider the environment and something that's not so harmful but at the same time it's efficient so I don't know all of them but I'm gonna go probably the natural gas, that looks
Interviewer : and why?
Maria : because well the name of it, first of all, is convincing, and it's, I feel like, I know gasoline is, can be harmful so, and I don't know so much about ethanol; I think methane could work
Interviewer : ok alright, so why did you decide then, it's the natural gas, because it has word natural in it?
Maria : yeah
The analysis of the above excerpt also reveals that Maria's reasoning was largely based on the direct association between naturalness of the source and level of pollution, without reference to a causal mechanism to justify such link. This is an example of “relational” reasoning as described in Table 2.
Maria also assumed that gaseous materials could cause more pollution, as gases get emitted into the air. As shown below, this association led her to question her initial decision:
Maria : well now after I see that the rest are liquid and this is the only one provided by gas, I'm thinking it's a good choice but then when I think about pollution and I'm just like, I don't know, there's just so many factors, I don't know
Interviewer : so you're sticking with the natural gas
Maria : yeah
Interviewer : and does the fact that it's a gas versus the other liquids have any impact on your choice?
Maria : it does, it has both, has a positive and a negative effect, has the negative effect because I'm thinking about pollution, I'm thinking about the air being polluted, I'm thinking about gas in the air and it's like, it's not a good thing, and then I'm thinking about how it's gas and it's less resources I'm using from, so yeah
Interviewer : ok so I just want to make sure I understand what you're saying. So because the natural is a gas it's gonna affect the air more?
Maria : yeah it could possibly affect the air more
As the third excerpt included below illustrates, although Maria had been exposed to introductory ideas about molecular structure and had learned enough basic organic nomenclature to associate names with key structural features, her final decision only involved considerations based on her two intuitive assumptions about the polluting nature of general types of materials (i.e., natural vs. artificial and gases vs. liquids):
Interviewer : when fuels are used in engines they can cause pollution, kind of like what you were saying, so in terms of how these four fuels would affect the environment, which of the fuels do you think would be better than others to use? as far as the amount of pollution
Maria : this is when I'm like stuck because I feel like natural gas, I don't even know so much about it, I feel like it, I don't know, I feel like it's good but then when I saw that it's a gas, it's the only one that's not a liquid, I, it's kind of tricky because I don't want it to affect so much pollution like I don't think it will affect as much, as the other ones, so I still I'm persistent, still sticking to my answer, I still choose natural gas because I feel like there's a clue in natural, in the way it, natural in the name I'm thinking it's used out of natural resources, things that are not harmful, so lot of good stuff in there
Interviewer : ok
Maria : yeah and E85 is ethanol, and you told us that the, when it has -ol at the end it's an alcohol and octane I'm guessing, isn't that it's something sweet in it? no, no, wait, no, the ending, I don't remember the ending
Interviewer : right we talked about it in [the] Smells [unit] ok
Interviewer : is there anything else that we should know about in making this decision that I haven't asked you yet?
Maria : I still stick to my choice and I feel like there has to be a clue in the word natural
Maria is a prototypical example of a student who does not express mechanistic reasoning in her justifications or explanations, but rather relies on simple associations to make her decisions (relational reasoning). Her thinking also illustrates the influence of intuitive assumptions about the properties of chemical entities. In particular, psychological research indicates that humans have a preference for what is perceived to be natural over what is perceived to be artificial (Rozin, 2005). Natural substances and processes are often linked to a subjective impression of goodness, while the products of human intervention are frequently judged more negatively. Positive feelings about entities or individuals are known to bias human decision-making (affect heuristic; Slovic et al., 2003), particularly in the absence of prior knowledge, or under conditions of limited time or low motivation.
In our second example, we present the case of a senior (fourth-year) university student (code name Cartesian), who was majoring in chemistry and had just recently completed a physical chemistry course. Cartesian's decisions were strongly influenced by the assumption that the best fuel would be the most available and easy to store and handle (because some sources were becoming exhausted, and because transportation and storage of the fuel could incur significant costs). His first choice was then ethanol, judged to be more abundant than the other fuels. He then switched to methane based on the assumption that gases were easier to store and transport. When asked about whether and how the molecular structures of the fuels were relevant to the choice of fuel, Cartesian expressed knowledge about structure–property relationships, but his reasoning was constrained by the search for the fuel that would be easier to store. His line of reasoning is illustrated in the following excerpt:
Interviewer : Okay. So now we're going to give you the structures of these molecules. Octane, methane, and ethanol. Do you think that knowing the structure is important and does that change your decision?
Cartesian : …(long pause)… I have to take a step back. I'm trying to be unpartisan because I know these are all sources. I took a bigger step back, knowing the structure would be an important element.
Cartesian : …(long pause)… I don't know how in-depth I should go, because we know that longer chains, you can look at the changes in boiling point and melting point and those types of areas. And you can try and maybe do an analysis of these will go at higher or lower temperatures and how much energy we would need to put in and out for the reactions to occur to be more cost efficient. But these are all pretty small, pretty similar compounds: octane, methane, and ethanol.
Interviewer : So from the structure you can determine melting point and boiling point and that would be important for combustion?
Cartesian : Yeah. You could think of it as kind of an… you could do it as an efficiency test. You could… I mean, longer chains you have more van der Waals and those types of forces, because these are all pretty similar compounds. Carbon chains, carbon chains, you have an alcohol group but that's not going to do very much. So I don't think any differences like that, for these compounds, would…I again don't think that would make too much of a difference. I think these are all pretty similar compounds for what we're using them for. I mean, you could look at the sizes and say …(pause)… we could look at the compounds and see the sizes and see how they stack if it would be more efficient for storage purposes. But methane being a gas, we could probably compress it a whole lot, so it's still probably the most efficient to store. So again, I don't really think the structures are too useful for this type of question.
Cartesian exhibited a higher level of conceptual sophistication than Maria, as he recognized relevant cues in making the targeted decision. Nevertheless, his chemical thinking was narrow and focused on physical properties of the materials. This was a common pattern observed in many of our interviews, where attention to common practical issues in the handling of substances (e.g., availability, storage, transport, safety, regular use) dominated over the comparative analysis of chemical properties. Cartesian's mode of reasoning was also more complex than Maria's. For example, in the previous excerpt his argument is multicomponent-integrated. He paid attention to a variety of factors, such as chemical composition, molecular size, and strength of intermolecular forces to build a causal mechanistic argument that could help him differentiate among the substances. However, the assumptions that guided his reasoning constrained his ability to pay attention to more relevant cues.
Our third and fourth cases illustrate the chemical thinking applied by two different experts in the discipline. The first of them (code name Aimake), was a professor with specialization in materials science and organic synthesis. In contrast with the previous two examples, his differentiation of matter types was based on a chemical argument. In particular, he considered that if complete combustion occurred, fuels could be differentiated by the oxidation state of carbon atoms in their molecules. If the combustion was incomplete, the outcome would depend on various other factors and would have to be empirically determined. The excerpt below illustrates his ideas:
Interviewer : Ok, last question. If your goal was to consider the environment, what would be best for the environment, which of these fuels would be best to use?
Aimake : That is a question that I would first make an assumption. Assume I have the most efficient engine I can get out there.
Interviewer : What do you mean by efficient?
Aimake : That it does complete combustion. If that's my hypothetical scenario, then I would still go for octane. Because for every carbon I use, I'm going to get more energy. And for every carbon, I get one CO 2 . If you use ethanol, which has less energy, I still get the CO 2 , but I get less energy. So assuming, to me, the chemically speaking, the byproduct is the same. You're going to get CO 2 . So, if I'm going to get CO 2 , I'd better get the maximum out of it. So it's a cost-benefit analysis. Because for every carbon there is, I'm getting the same molecule, the same product. It doesn't matter. But, if I'm working now with an inefficient engine, then I have to worry, oh, am I getting complete combustion? Or am I getting these other highly active molecules, sub-… not fully oxidized carbon products that could be really detrimental to the environment. Then in that case, when I have inefficient engine, then I have to consider the actual products themselves, which right now I don't know. But in a case where I have a complete ideal, I would still go for octane.
The second professor (code name Caesar), with specialization in catalysis and medicinal chemistry, and who had worked in the paint industry, relied on different cues in his decision. For this individual, the source of carbon in fuels mattered. He assumed that recycling carbon atoms was less damaging to the environment than pumping new carbon into the atmosphere, therefore ethanol from a biological source (fermentation of sugars) was better than octane from petroleum, but ethanol from petroleum (via cracking) would not be better than octane from the same source. The excerpt below illustrates his argument:
Interviewer : Our last question is concerned with pollution. If the goal is to reduce pollution, which would be the best fuel?
Caesar : No question, ethanol.
Interviewer : Why?
Caesar : Because you are recycling carbon atoms. You see the cane sugar, or whatever was the source, the biological source, where ethanol comes. That is, ethanol is from a biological, a primary biological source, then we will be just recycling. If we, you would say, in petroleum we have ethane (writes C 2 H 6 ). It can be cracked to ethylene (draws arrow, then C 2 H 4 + H2). And part of it could be hydrated to ethanol (draws arrow from C2H4to EtOH, writes H2O beside arrow). If this is the source of ethanol, then we're just pumping CO2into the atmosphere. But if ethanol comes from a biological source, then we are just recycling carbon atoms… So it has to do with the origin of the ethanol. If it comes from a petroleum source, we will keep on pumping carbon into the atmosphere. But if it comes from a biological source, then we'll just be recycling carbon. And so if you assure me that the ethanol would come from sugars, from the fermentation of sugars, that would be okay.
Both Aimake and Caesar cued on chemical composition issues in their analysis, but focused their attention on different aspects of the problem. Aimake paid attention to the ratio of energy to CO2 produced based on the analysis of the oxidation state of carbon atoms in each fuel, while Caesar cued on the origin of such atoms. Both professors displayed high conceptual sophistication in their chemical thinking, although most of their reasoning was expressed at a relational level. For example, initially Aimake simply associated the amount of energy produced per carbon atom to the oxidation state of such atoms, without reference to any causal mechanism to justify his argument. Similarly, Caesar claimed that recycled carbons were better than new carbon atoms for the environment, without further explanation. The assumptions that these individuals made led them to rely on relevant chemical cues in making their decision, but their expressed reasoning was highly associative, relying on knowledge that was not explicitly stated. Nevertheless, both of experts were able to adopt a multicomponent-integrated mode of reasoning when prompted.
The examples presented in this section demonstrate that in order to characterize differences in people's chemical thinking it is important to pay attention to both the assumptions (whether more intuitive or more academic) that they rely upon, as well as the modes of reasoning that they employ. The previous excerpts illustrate how a novice student cued on features that were not centrally relevant, using relational or linear causal reasoning to justify her decisions. Experts applied similar modes of reasoning, but they cued on critical factors, building valid associations that were justified by knowledge that can be made explicit. The student at an intermediate stage relied on multicomponent reasoning, but struggled to discriminate among relevant and irrelevant cues in making his decisions. The assumptions that he made were hybrids, involving intuitive ideas mixed with academic constructs. Characterizing pathways of progression may thus be challenging given the diversity of ideas that intermediate students often bring into their analyses.
As the GoKart instrument illustrates, we are exploring students' chemical thinking in terms of their conceptual sophistication and modes of reasoning, using semi-open investigation, design, or evaluation tasks that engage students in one or more core practices (analysis, synthesis, transformation) and pose questions that allows us to elicit how students apply their knowledge in relation to relevant crosscutting disciplinary concepts. As seen in the examples, participants cue on different factors, often in relation to different crosscutting concepts. For example, Maria cued on potential harm to the environment (benefits–costs–risks), while Caesar cued on the method and source of production (chemical mechanism). Our approach thus demands the design and development of an assortment of research instruments that allow us to map the complex landscape of chemical thinking (NRC, 2001).
Final comments
Our work in the development and validation of the CTLP represents, for us, a means to rethink both chemistry education and chemistry education research in a way that is more authentic to our discipline and more meaningful and relevant to students. Our efforts provide an alternative lens through which to analyze and think about chemistry teaching and learning (Talanquer, 2013c). We are carrying out this work involving and seeking the input of critical stakeholders. First, the CTLP must be true to the discipline of chemistry. Therefore, we are asking expert chemists in academia, industry, and government and regulatory sectors to help us validate important components of the LP, such as the essential questions that define our progress variables and the associated upper anchors. Second, we are working with secondary school science teachers who are not only collaborating with us in the design of research instruments and the collection research data, but are also helping us ground our development in the authenticity of real classrooms. Third, our studies involve students from a variety of educational levels and cultural and economic backgrounds. Our goal is to explore how chemical thinking develops within existing learning conditions, as well as how it could progress when considering the alternative pedagogies that we advocate. Finally, we recognize that our educational and research pursuits exist in the context of national frameworks and standards, and thus our CTLP should be responsive to them but also challenge the status quo (Talanquer and Sevian, 2013).We expect that our efforts will result in tangible products to support and facilitate chemistry teaching and learning driven by our CTLP. We are currently developing detailed descriptions of the learning progression from novice (lower anchor) to sophisticated (upper anchor) chemical thinking along all six of the crosscutting disciplinary concepts. Such descriptions include careful articulation of productive intermediate understandings (stepping stones) that support the reconceptualization of chemistry ideas. These descriptions are characterized in terms of core assumptions and modes of reasoning that have emerged from our analysis. We are also collaborating with teachers in our research team to create instruments that can be used both as research tools and as formative assessments in the classroom. Our intent is to produce instruments that will give teachers relatively rapid and valuable feedback on the assumptions and modes of reasoning that constrain their students' thinking, and provide guidance on how to scaffold student learning. These instruments are taking a wide range of forms, including two-tiered tests, questionnaires with open-ended questions, cognitive interviews, and teaching experiments with specific prompts.
We are strong advocates of educational and research efforts that seek to create conditions for students to actively engage in relevant, meaningful, and productive ways of thinking in the science classroom. Nevertheless, we believe that such educational approaches should emerge from a critical analysis of the goals, practices, and ways of thinking that characterize each scientific discipline. They should also be responsive to the role and impact that the products of such human enterprise have at the personal, societal, and global levels. Equally important, they should be based on the careful investigation and analysis of how students' assumptions and modes of reasoning progress over time under various conditions. The work described in this paper seeks to contribute to such types of efforts.
Acknowledgements
The authors wish to acknowledge the funding sources, US NSF awards DRL-1221494 and DRL-1222624, and a AAAS Women's International Research Collaborations at Minority-Serving Institutions award, that support our work. We thank all of the graduate students and chemistry teachers who collaborate with us in the development of the CTLP. We would also like to give credit to Jason Green for allowing us to use Fig. 1. Any opinions, conclusions, or recommendations expressed in this paper are those of the authors, and do not necessarily reflect the views of the funding sources.References
- Alonzo A. and Steedle J. T., (2009), Developing and assessing a force and motion learning progression, Sci. Educ., 93(3), 389–421.
- Andersson B., (1986), The experimental gestalt of causation: a common core to pupils' preconceptions in science, Eur. J. Sci. Educ., 8(2), 155–171.
- Andersson B., (1990), Pupils' conceptions of matter and its transformations (age 12–16), Stud. Sci. Educ., 18, 53–85.
- Atkins P. and de Paula J., (2006), Physical Chemistry, 8th edn, Oxford: Oxford University Press.
- Au K. T., (1994), Developing an intuitive understanding of substance kinds, Cognitive Psychol., 27, 7–111.
- Bensaude-Vincent B. and Simon J., (2008), Chemistry: the impure science, London: Imperial College Press.
- Berland L. K. and McNeill K. L., (2010), A learning progression for scientific argumentation: understanding student work and designing supportive instructional contexts, Sci. Educ., 94, 765–793.
- Bernholt S. and Parchmann I., (2011), Assessing the complexity of students' knowledge in chemistry, Chem. Educ. Res. Pract., 12, 167–173.
- Bhattacharyya G. and Bodner G. M., (2005), It gets me to the product: how students propose organic mechanisms, J. Chem. Educ., 82, 1402–1407.
- Biggs J. B. and Collis K. F., (1982), Evaluating the quality of learning: the SOLO taxonomy (Structure of the Observed Learning Outcome), New York: Academic Press.
- Breslow R., (1997), Chemistry today and tomorrow: the central, useful, and creative science, Washington, D.C.: American Chemical Society.
- Brown D. E. and Hammer D., (2008), Conceptual change in physics, in Vosniadou S. (ed.), International handbook of research on conceptual change, New York: Routledge, pp. 127–154.
- Brown N. J. S., Nagashima S. O., Fu A., Timms M. and Wilson M., (2010), A framework for analyzing scientific reasoning in assessments, Educational Assessment, 15(3), 142–174.
- Bulte A. M. W., Westbroek H. B., De Jong O. and Pilot A., (2006), A research approach to designing chemistry education using authentic practices as contexts, Int. J. Sci. Educ., 28(10), 1063–1086.
- Bybee R. W., (1987), Science Education and the Science-Technology-Society (S-T-S) Theme, Sci. Educ., 71(5), 667–683.
- Bybee R., (1997), Achieving scientific literacy, Portsmouth, NH: Heinemann.
- Bybee R., Taylor J. A., Gardner A., Van Scotter P., Carlson J., Westbrook A. and Landes, N., (2006), The BSCS 5E instructional model: origins and effectiveness, Colorado Springs, CO: BSCS.
- Carroll F. A., (1998), Perspectives on structure and mechanism in organic chemistry, Pacific Grove, CA: Brooks/Cole.
- Chamizo J. A., (2013), Technochemistry: one of the chemists' ways of knowing, Found. Chem., 15, 157–170.
- Chi M. T. H., (2005), Commonsense conceptions of emergent processes: why some misconceptions are robust, J. Learn. Sci., 14(2), 161–199.
- Chi M. T. H., (2008), Three kinds of conceptual change: belief revision, mental model transformation, and ontological shift, in Vosniadou S. (ed.), International handbook of research on conceptual change, New York: Routledge, pp. 61–82.
- Claesgens J., Scalise K., Wilson M. and Stacy A., (2009), Mapping student understanding in chemistry: the perspectives of chemists, Sci. Educ., 93, 56–85.
- Cooper M. M., Corley L. M. and Underwood S. M., (2013), An investigation of college chemistry students' understanding of structure–property relationships, J. Res. Sci. Teach., 50(6), 699–721.
- Cooper M. M., Underwood S. M., Hilley C. Z. and Klymkowsky M. W., (2012), Development and assessment of a molecular structure and properties learning progression, J. Chem. Educ., 89, 1351–1357.
- Corcoran T., Mosher F. A. and Rogat A., (2009), Learning progressions in science: an evidence-based approach to reform, Consortium for Policy Research in Education Report #RR-63. Philadelphia, PA: Consortium for Policy Research in Education.
- diSessa A. A., (1993), Toward an epistemology of physics, Cognition Instruct., 10, 165–255.
- diSessa A. A., (2008), A bird's eye view of the ‘pieces’ versus ‘coherence’ controversy (from the ‘pieces’ side of the fence), in Vosniadou S. (ed.), International handbook of research on conceptual change, New York: Routledge, pp. 35–60.
- Dugger W. E., (2001), Standards for technological literacy, Phi Delta Kappan, 82, 513–517.
- Duncan R. G. and Rivet A. E., (2013), Science learning progressions, Science, 339(6118), 396–297.
- Duncan R. G., Rogat A. and Yarden, A., (2009), A learning progression for deepening students' understandings of modern genetics across the 5th–10th grades, J. Res. Sci. Teach., 46, 655–674.
- Duschl R., Maeng S. and Sezen A., (2011), Learning progressions and teaching sequences: a review and analysis, Stud. Sci. Educ., 47(2), 123–182.
- Eilks E., Rauch F., Ralle B. and Hofstein A., (2013), How to allocate the chemistry curriculum between science and society, in Eilks I. and Hofstein A. (ed.), Teaching chemistry – a studybook, Rotterdam, The Netherlands: Sense Publishers.
- Eisenkraft E., (2003), Expanding the 5E model, Sci. Teach., 70(6), 56–59.
- Enke C. G., (2001), The art and science of chemical analysis, New York, NY: Wiley.
- French M., (1999), Conceptual design for engineers, London: Springer.
- Gilbert J. K. and Treagust D. (ed.), (2009), Multiple representations in chemical education, The Netherlands: Springer.
- Gilbert J. K., De Jong O., Justi R., Treagust D. and van Driel J. (ed.), (2002), Chemical education: towards research-based practice, Dordrecht: Kluwer.
- Grotzer T. A., (2003), Learning to understand the forms of causality implicit in scientifically accepted explanations, Stud. Sci. Educ., 39, 1–74.
- Hesse J. J. and Anderson C. W., (1992), Students' conceptions of chemical change, J. Res. Sci. Teach., 29(3), 277–299.
- Hoffmann R., (1993), How should chemists think? Sci. Am., 268(2), 66–73.
- Johnson P., (2000), Children's understanding of substances, part 1: recognizing chemical change, Int. J. Sci. Educ., 22(7), 719–737.
- Johnson P., (2002), Children's understanding of substances, part 2: explaining chemical change, Int. J. Sci. Educ., 24(10), 1037–1054.
- Johnson P. and Tymms P., (2011), The emergence of a learning progression in middle school chemistry, J. Res. Sci. Teach., 8(8), 849–877.
- Kahan D. M., Jenkins-Smith H. and Braman D., (2011), Cultural cognition of scientific consensus, J. Risk Res., 14, 147–174.
- Kind V., (2004), Beyond appearances: students' misconceptions about basic chemical ideas, 2nd edn, London: Royal Society of Chemistry.
- King D., (2012), New perspectives on context-based chemistry education: using a dialectical sociocultural approach to view teaching and learning, Stud. Sci. Educ., 48(1), 51–87.
- Lacy S., Tobin R., Wiser M. and Crissman S., (2012), Looking through the energy lens: a proposed learning progression for energy in grades 3–5, Paper presented at the Energy Summit, 2012, http://esummit-msu.net/content/looking-through-energy-lens-proposed-learning-progression-energy-grades-3-5, accessed on August 27, 2013.
- Lehrer R. and Schauble L., (2012), Seeding evolutionary thinking by engaging children in modeling its foundations, Sci. Educ., 96(4), 701–724.
- Levy S. T. and Wilensky U., (2009), Crossing levels and representations: the connected chemistry (CC1) curriculum, J. Sci. Educ. Technol., 18(3), 224–242.
- Liu X. and Lesniak K., (2005), Students' progression of understanding the matter concept from elementary to high school, Sci. Educ., 89(3), 433–450.
- Maeyer J. and Talanquer V., (2010), The role of heuristics in students thinking: ranking of chemical substances, Sci. Educ., 94(6), 963–984.
- Maeyer J. and Talanquer V., (2013), Making predictions about chemical reactivity: assumptions and heuristics, J. Res. Sci. Teach., 50(6), 748–767.
- Mohan L., Chen J. and Anderson C. W., (2009), Developing a multi-year learning progression for carbon cycling in socio-ecological systems, J. Res. Sci. Teach., 46(6), 675–698.
- National Research Council (NRC), (1996), The national science education standards, Washington, D.C.: National Academies Press.
- National Research Council (NRC), (2001), Knowing what students know: the science and design of educational assessment, Washington, D.C.: National Academy Press.
- National Research Council (NRC), (2003), Beyond the molecular frontier: challenges for chemistry and chemical engineering, Washington, D.C.: National Academy Press.
- National Research Council (NRC), (2007), Taking science to school: learning and teaching science in grades K-8, Washington, D.C.: National Academies Press.
- National Research Council (NRC), (2011), A framework for K-12 science education: practices, crosscutting concepts, and core ideas, Washington, D.C.: The National Academies Press.
- National Research Council (NRC), (2013), The next generation science standards, Washington, D.C.: The National Academies Press.
- Osborne J. F. and Dillon J., (2008), Science Education in Europe, London: Nuffield Foundation.
- Padilla M. J., (1986), The science process skills, Research matters…to the science teacher, Archived by NARST as No. 9004, March 1, 1990, http://www.narst.org/publications/research/skill.cfm, accessed on August 1, 2013.
- Padilla M., Okey J. and Dillashaw F., (1983), The relationship between science process skills and formal thinking abilities, J. Res. Sci. Teach., 20(3), 239–246.
- Redish E. F., (2004), A theoretical framework for physics education research: modeling student thinking, in Redish E. F. and Vicentini M. (ed.), Proceedings of the International School of Physics, “Enrico Fermi” Course CLVI, Amsterdam: IOS Press.
- Rozin P., (2005), The meaning of natural, Psychol. Sci., 16(8), 652–658.
- Sadler T. D. and Donnelly L. A., (2006), Socio-scientific argumentation: the effects of content knowledge and morality, Int. J. Sci. Educ., 28, 1463–1488.
- Sadler T. D. and Fowler S., (2006), A threshold model of content knowledge transfer for socio-scientific argumentation, Sci. Educ., 90, 986–1004.
- Schecker H. and Parchmann I., (2007), Standards and competence models: The German situation, in Waddington D., Nentwig P. and Schanze S. (ed.), Standards in science education, Münster, Germany: Waxmann, pp. 147–164.
- Schwartz A. T., (2006), Contextualized chemistry education: the American experience, Int. J. Sci. Educ., 28, 977–998.
- Sevian H. and Stains M. N., (2013), Implicit assumptions and progress variables in a learning progression about structure and motion of matter, in Tsaparlis G. and Sevian H. (ed.), Concepts of matter in science education, Dordrecht: Springer, pp. 69–94.
- Sikorski T. F. and Hammer D., (2010), A critique of how learning progressions research conceptualizes sophistication and progress, in Gomez K., Lyons L. and Radinsky J. (ed.), Learning in the Disciplines: Proceedings of the 9th International Conference of the Learning Sciences, Chicago, IL: International Society of the Learning Sciences, vol. 1, pp. 1032–1039.
- Sjöström J., (2013), Towards Bildung-oriented chemistry education, Sci. & Educ., 22(7), 1873–1890.
- Slovic P., Finucane M., Peters E. and MacGregor D. G., (2003), The affect heuristic, in Gilovich T., Griffin D. and Kahneman D. (ed.), Intuitive judgment: heuristics and biases, Cambridge University Press, pp. 397–420.
- Smith C., Carey S. and Wiser M., (1985), On differentiation: a case study of the development of size, weight, and density, Cognition, 21, 177–237.
- Smith C., Wiser M., Anderson C. and Krajcik J., (2006), Implications of research on children's learning for standards and assessment: a proposed learning progression for matter and atomic-molecular theory, Measurement, 14(1&2), 1–98.
- Smith C. L., Wiser M. and Carraher D. W., (2010), Using a comparative, longitudinal study with upper elementary school students to test some assumptions of a learning progression for matter, Paper presented at the annual meeting of the National Association for Research on Science Teaching, Philadelphia, PA.
- Solomon J., (1996), Teaching science, technology and society: developing science and technology series, Bristol, PA: Taylor and Francis.
- Spelke E. S. and Kinzler K. D., (2007), Core knowledge, Dev. Sci., 10, 89–96.
- Stains M. N. and Sevian H., (2013), Uncovering implicit assumptions: a large-scale study on students' mental models of diffusion, J. Learn. Sci., under review.
- Stevens S., Delgado C. and Krajcik J. S., (2010), Developing a hypothetical multi-dimensional learning progression for the nature of matter, J. Res. Sci. Teach., 47, 687–715.
- Taber K., (2009), College students' conceptions of chemical stability: the widespread adoption of a heuristic rule out of context and beyond its range of application, Int. J. Sci. Educ., 31(10), 1333–1358.
- Taber K. S., (2013), A common core to chemical conceptions: learners' conceptions of chemical stability, change and bonding, in Tsaparlis G. and Sevian H. (ed.), Concepts of matter in science education, Dordrecht: Springer, pp. 391–418.
- Taber K. S. and García-Franco A., (2010), Learning processes in chemistry: drawing upon cognitive resources to learn about the particulate structure of matter, J. Learn. Sci., 19(1), 99–142.
- Talanquer V., (2006), Common sense chemistry: a model for understanding students' alternative conceptions, J. Chem. Educ., 83(5), 811–816.
- Talanquer V., (2008), Students' predictions about the sensory properties of chemical compounds: additive versus emergent frameworks, Sci. Educ., 92(1), 96–114.
- Talanquer V., (2009), On cognitive constraints and learning progressions: the case of structure of matter, Int. J. Sci. Educ., 31(15), 2123–2136.
- Talanquer V., (2009), On cognitive constraints and learning progressions: the case of “structure of matter”, Int. J. Sci. Educ., 31, 2123–2136.
- Talanquer V., (2010), Exploring dominant types of explanations built by general chemistry students, Int. J. Sci. Educ., 32(18), 2393–2412.
- Talanquer V., (2013a), School chemistry: the need for transgression, Sci. & Educ., 22, 1757–1773.
- Talanquer V., (2013b), How do students reason about chemical substances and reactions? in Tsaparlis G. and Sevian H. (ed.), Concepts of matter in science education, Dordrecht: Springer, pp. 331–346.
- Talanquer V., (2013c), Chemistry education: ten facets to shape us, J. Chem. Educ., 90(7), 832–838.
- Talanquer V. and Pollard J., (2010), Let's teach how we think instead of what we know, Chem. Educ. Res. Pract., 11, 74–83.
- Talanquer V. and Sevian H., (2013), Chemistry in past and new science frameworks and standards: gains, losses, and missed opportunities, J. Chem. Educ., DOI: 10.1021/ed400134c.
- Van Berkel B., de Vos W., Verdonk A. H. and Pilot A., (2000), Normal science education and its dangers: the case of school chemistry, Sci. & Educ., 9, 123–159.
- Vilches A. and Gil-Pérez D., (2013), Creating a sustainable future: some philosophical and educational considerations for chemistry teaching, Sci. & Educ., 22(7), 1857–1872.
- von Aufschnaiter C. and von Aufschnaiter S., (2003), Theoretical framework and empirical evidence of students' cognitive processes in three dimensions of content, complexity, and time, J. Res. Sci. Teach., 40(7), 616-648.
- Vosniadou S., (1994), Capturing and modeling the process of conceptual change, Learn. Instr., 4, 45–69.
- Vosniadou S. and Ortony A. (ed.), (1989), Similarity and analogical reasoning, New York: Cambridge University Press.
- Vosniadou S., Vamvakoussi X. and Skopeliti I., (2008), The framework theory approach to the problem of conceptual change, in Vosniadou S. (ed.), International handbook of research on conceptual change, New York: Routledge, pp. 3–34.
- Waddington D., Nentwig P. and Schanze S. (ed.), (2007), Standards in science education, Münster, Germany: Waxmann.
- Wilson M., (2009), Measuring progression: assessment structures underlying a learning progression, J. Res. Sci. Teach., 46, 716–730.
- Wiser M. and Smith C. L., (2008), Learning and teaching about matter in Grades K-8: when should the atomic-molecular theory be introduced? in Vosniadou S. (ed.), International Handbook of Research on Conceptual Change, New York: Routledge, pp. 205–239.
- Wiser M., Fox V. and Frazier K., (2013), At the beginning was amount of material: a learning progression for matter for early elementary grades, in Tsaparlis G. and Sevian H. (ed.), Concepts of matter in science education, Dordrecht: Springer, pp. 95–122.
- Zeidler D. L., Sadler T. D., Simmons M. L. and Howes E. V., (2005), Beyond STS: a research-based framework for socio-scientific issues education, Sci. Educ., 89, 357–377.
| This journal is © The Royal Society of Chemistry 2014 |