PEGylated CsxWO3 nanorods as an efficient and stable 915 nm-laser-driven photothermal agent against cancer cells
Wenju Xu,
Zhouqi Meng,
Nuo Yu,
Zhigang Chen*,
Bin Sun,
Xiaoze Jiang and
Meifang Zhu*
State Key Laboratory for Modification of Chemical Fibers and Polymer Materials, College of Materials Science and Engineering, Donghua University, Shanghai 201620, China. E-mail: zgchen@dhu.edu.cn; zmf@dhu.edu.cn
First published on 15th December 2014
Abstract
WO3−x nanomaterials have been demonstrated to be one kind of efficient near-infrared (NIR) laser-driven photothermal nanoagents, but their photothermal stability is still unsatisfied. In addition, a 980 nm laser is usually used as NIR light source, but it has an overheating effect due to optical absorption of water and biological specimens. To address these problems, we have prepared PEGylated Cs-doped WO3 (CsxWO3) nanorods by a solvothermal synthesis—PEGylation two-step route. CsxWO3 nanorods have diameters of ∼11 nm and lengths of ∼50 nm, and they exhibit increased absorption in the NIR region (700–1100 nm). With PEGylated CsxWO3 nanorods as the photothermal nanoagent, we compare the overheating and penetration effects of 915 and 980 nm lasers as NIR light sources. Compared with the 980 nm laser, the 915 nm laser provides drastically less overheating of water, and higher penetration ability of water/skin due to quite low water absorption. Importantly, under the irradiation of a 915 nm laser, CsxWO3 nanorods exhibit excellent photothermal conversion performance with high stability. Furthermore, by the photothermal effect of PEGylated CsxWO3 nanorods, in vivo cancer cells can be efficiently destroyed under the irradiation of a 915 nm laser. Therefore, PEGylated CsxWO3 nanorods can be used as a promising efficient and stable NIR-laser-driven photothermal agent against in vivo cancer cells.
1. Introduction
Near-infrared (NIR, λ = 700–1100 nm) laser-induced photothermal ablation (PTA) has drawn much interest, since it is a minimally invasive and potentially more effective therapeutic technology for cancer.1 To promote the photothermal conversion efficiency and to improve the lasers' discrimination, a prerequisite for the development of NIR-PTA is to obtain efficient, stable and biocompatible photothermal agents.2 To address this problem, different photothermal agents have been developed, including polymer nanoparticles,3,4 noble metal nanostructures,5,6 carbon-based nanomaterials7,8 and semiconductor nanomaterials.9,10 Among these photothermal agents, semiconductor nanomaterials have attracted increasing attention of researchers, due to their low cost, high conversion efficiency and low cytotoxicity. Currently, there are chiefly two kinds of semiconductor photothermal nanoagents. One kind is copper-based nanomaterials, including CuS1−x9 and CuSe.11 We have also developed several kinds of CuS1−x-based nanomaterials, such as CuS superstructures,2 Cu9S5 nanoparticles,12 Cu9S5@mSiO213 and Fe3O4@Cu2–xS core–shell nanoparticles.14 The other kind is W-based nanomaterials, including W18O49 nanowires,15 WO2.9 nanorods16 and WS2 nanosheets.17 However, the properties (such as photothermal efficiency, stability and cytotoxicity, etc.) of these photothermal nanoagents are still unsatisfied to meet the future biomedical requirements. Therefore, new kinds of photothermal agents should be further developed.It is well-known that the photothermal performances of nanoagents are seriously dependent on their compositions, structures, morphologies and sizes.18,19 Recently, to improve the photothermal performance of W18O49 nanowires,15 we optimized the length of nanowires, and W18O49 nanowires with average lengths of about 800 nm exhibited better NIR photoabsorption and photothermal conversion performance than W18O49 short nanowires with average lengths of about 50 nm.20 However, we also found that the NIR photoabsorption and photothermal performance of W18O49 nanowires can not be well remained when the aqueous dispersion was stored for several weeks, particularly at high temperature under O2 atmosphere. The origin of NIR photoabsorption of WO3−x can be considered to be closely related to the free electrons and/or oxygen-deficiency-induced small polarons.21 The oxygen-deficiency-induced small polarons and the metastable crystalline phase can be destroyed by the oxidant (such as H2O2),22 resulting in the long-term instability. Thus, it remains a great challenge to prepare WO3−x-based nanoagents with high photothermal efficiency and excellent stability.
As we know, WO3−x nanoagents could be doped with metal ions (including Cs+, B3+, Y3+ and so on)23–26 to improve their physical performance. The metal ions may donate one electron to W6+ in WO3 to form W5+ ions,27 and these W5+ ions form small to medium size polarons which are more stable than the oxygen-deficiency-induced small polarons in WO3−x. Until now there are few researches of doped WO3−x nanoagents on biomedical aspects.28 In the present work, to improve the stability and remain excellent photothermal performance, we prepared PEGylated Cs-doped WO3 (CsxWO3) nanorods by the solvothermal synthesis—PEGylation two-step route. With PEGylated CsxWO3 nanorods as the photothermal nanoagent, the overheating and penetration effects of 915 and 980 nm lasers were compared, and the results revealed that 915 nm laser should be better NIR light source. Under the irradiation of 915 nm laser, CsxWO3 nanorods exhibited excellent photothermal performance and high stability, resulting in the efficient PTA of in vivo cancer cells.
2. Materials and methods
2.1 Materials
Absolute ethanol, acetic acid, poly(ethylene glycol) (molecular weight MW = 400 Da, abbreviated as PEG-400) were purchased from Sinopharm Chemical Reagent Co., Ltd. Tungsten hexachloride (WCl6) and cesium hydroxide monohydrate (CsOH·H2O) were purchased from J&K Scientific Ltd. 2-[Methoxy(polyethyleneoxy) propyl]trimethoxysilane (PEG-silane, molecular weight: 460–590 g mol−1) was obtained from Gelest (Morrisville, PA). Dimethyl sulfoxide (DMSO) and 3-(4,5)-dimethylthiahiazo-2-yl-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma-Aldrich Co. Ltd.2.2 Synthesis of PEGylated CsxWO3 nanorods and W18O49 nanowires
PEGylated CsxWO3 nanorods were prepared by the solvothermal synthesis—PEGylation two-step route. In the first step, CsxWO3 nanorods without any hydrophilic polymer ligands were obtained by a modified solvothermal route.29 In a typical process, WCl6 (0.25 g, 0.6 mmol) and CsOH·H2O (0.08 g, 0.5 mmol) were dissolved in ethanol (40 mL), and then 10 mL acetic acid was added. The resulting solution was stirred for several minutes and subsequently transferred into a Teflon-lined stainless steel autoclave, sealed and treated at 180 °C for 24 hours. Blue CsxWO3 sample was collected by centrifugation and washed with ethanol for several times. To improve the biocompatibility, in the second step, CsxWO3 sample was dispersed in deionized water and 200 μL PEG-silane was added to the as-formed dispersion, and then the dispersion was stirred for 3 h. The final precipitate was collected by centrifugation, washed with deionized water for several times. Part of the final precipitate was dried in vacuum at 50 °C for further character analysis, and part of the final precipitate was directly dispersed in deionized water to form a high concentration aqueous dispersion for dilution after quantitative measurement.For stability comparison, PEGylated W18O49 nanowires were also prepared via a modified solvothermal route by treating an ethanol and PEG-400 hybrid solution (volume ratio: 3/7) containing WCl6 at 180 °C for 24 h, according to our previous report.15
2.3 Characterization and photothermal measurement
Morphologies of CsxWO3 nanorods before and after PEG-silane treatment were determined by high-resolution transmission electron microscope (HR-TEM; JEOL JEM-2010F). The crystal phase was determined by X-ray diffraction (XRD) analysis (Bruker D4 X-ray diffractometer) using graphite-monochromatized Cu Kα radiation (λ = 0.15418 nm). The elementary composition of the sample was characterized by an energy-dispersive X-ray spectrometer (EDS) on a Bruker Quantax 400 EDS system attached to HR-TEM (JEOL JEM-2010F). The presence of PEG-silane was determined by the Fourier transform infrared (FT-IR) spectrum in KBr pellets (IRPRESTIGE-21-Shimadzu). Photoabsorption spectrum of the aqueous dispersion containing PEGylated CsxWO3 nanorods was measured on a Shimadzu UV-2550 UV-visible-NIR spectrophotometer using a quartz cuvette with an optical path of 10 mm.To investigate the overheating and penetration effects of NIR laser, 915 and 980 nm laser (Shanghai Xilong Optoelectronics Technology Co. Ltd, China) were used as light sources. A quartz cuvette (optical path: 5 mm) containing pure water (0.3 mL, depth 5 mm) or the PEGylated CsxWO3 aqueous dispersion (0.3 mL, 0.25 mg mL−1) was directly illuminated by 980/915 nm laser with the power density of 0.72 W cm−2 in the absence of any barrier in laser pathway. The temperature of the water or PEGylated CsxWO3 aqueous dispersion was real-time recorded by an infrared thermal imaging camera (A300, FLIR systems Inc.). Subsequently, pure water (1 mL, in a quartz cuvette with an optical path of 10 mm) or chicken skin (thickness: about 1 mm) was placed in front of aqueous dispersion containing PEGylated CsxWO3 as a barrier layer in laser pathway, and the temperature of aqueous dispersion was real-time recorded again under the irradiation of 980/915 nm laser with the power density of 0.72 W cm−2.
To investigate the effect of CsxWO3 concentrations on the temperature, a plastic tube containing CsxWO3 solution (0.1 mL) with different concentration (0–1.0 mg mL−1) was illuminated by 915 nm laser with the power density of 0.72 W cm−2. The temperatures were real-time recorded by an infrared thermal imaging camera.
To study the photothermal stability, the aqueous dispersion (0.3 mL) containing PEGylated CsxWO3 nanorods or W18O49 nanowires with the same concentration (1.0 mg mL−1) was illuminated by 915 nm laser with the power density of 0.72 W cm−2, and the temperature was determined once every 24 h in a week. The temperatures of the aqueous dispersions were real-time recorded by an infrared thermal imaging camera.
2.4 Cytotoxicity assay in vitro and PTA of cancer tissues in vivo
The in vitro cytotoxicity of PEGylated CsxWO3 nanorods was determined by using a MTT assay in the human cervical carcinoma cell line HeLa. The HeLa cells were seeded into a 96-well tissue culture plate at a density of 5 × 103 cells per well for 24 h at 37 °C in 5% CO2. After 24 h incubation, the culture medium was replaced and cells were incubated with complete medium containing PBS or the PEGylated CsxWO3/PBS dispersions with different concentrations at 37 °C in 5% CO2 for another 24 h. Then 100 μL per well of MTT solution (1 mg mL−1) was added into the microtiter plate and then was incubated in the CO2 incubator for 4 h. The cells then were lysed by the addition of 150 μL DMSO and shaken by shaking table for 10 min. The absorbance of formazan was measured using a plate reader at 570 nm.The PTA of cancer cells in vivo was carried out by a modified method, according to our previous studies.2,14,20 Severe combined immunodeficiency (SCID) mice were inoculated subcutaneously with 2 × 106 human breast cancer MAD-MB-231cells in the right side of the rear for 21 days. The SCID mice were randomly assigned into two groups as control and treatment groups (n = 5 per group) after tumors inside the mice grew to 5–10 mm in diameter. The SCID mice in the treatment group were injected with 100 μL of PBS dispersion containing PEGylated CsxWO3 nanorods (0.25 mg mL−1) at the central region of the tumor with a depth of ∼4 mm, while the SCID mice in the control group were injected with 100 μL saline solution. Then the mice with tumors of the control and treatment groups were irradiated by two similar 915 nm laser devices with 0.72 W cm−2 for 10 min in batches. The SCID mice were killed after 24 hours, and tumors were removed, embedded in paraffin, and cryosectioned into 4 μm slices. The slides were stained with hematoxylin/eosin. The slices were examined under an inverted fluorescence microscope.
3. Results and discussion
3.1 Synthesis and characterization of PEGylated CsxWO3 nanorods
The CsxWO3 nanorods were prepared by a modified solvothermal process,29 where WCl6, CsOH·H2O and acetic acid were added into ethanol solution and solvothermally treated at 180 °C for 24 h (step I in Fig. 1). Fig. 2a and b shows TEM and HR-TEM images of the as prepared sample. Obviously, CsxWO3 sample consists of nanorods with diameters of ∼11 nm and lengths of ∼50 nm. Furthermore, the high-resolution TEM image (the inset in Fig. 2b) also indicates clear lattice fringes, suggesting that the nanorod is a single crystal. The interplanar d-spacing is determined to be 0.37 nm, which agrees well with the (002) lattice fringes of hexagonal phase Cs0.32WO3.28 This fact also suggests that the nanorod grows along the c-axis of the hexagonal tungsten bronze structure, which is similar to the previous reports.30 | ||
| Fig. 1 Schematic illustration of showing the solvothermal synthesis—PEGylation two-step route for the synthesis of PEGylated CsxWO3 nanorods. | ||
To improve the water dispersibility and biocompatibility, in the present study, PEG-silane was added to CsxWO3 nanorods dispersion and the hybrid dispersion was stirred for 3 h (step II in Fig. 1). After PEGylation treatment, CsxWO3 nanorods still remain the diameter of ∼11 nm and length of ∼50 nm (Fig. 2c and d), indicating no obvious morphology change. This fact is different from simultaneous PEGylation/exfoliation/breaking process of W18O49 nanowires,20 and may result from that the diameter (∼11 nm) of CsxWO3 nanorods is large greatly compared to that (∼0.9 nm) of W18O49 nanowires, and nanomaterials with large diameter are not easy to break.
The crystal phase and composition of PEGylated CsxWO3 nanorods was further characterized. XRD pattern (Fig. 3a) reveals that all peaks could be well indexed to the hexagonal cesium tungsten bronze Cs0.32WO3 phase (JCPDS no. 83-1334) and no impurity peak was observed. The intensity is found to be high and peak width to be narrow in accordance with the general crystalline features of nanocrystals. This result agrees well with the previous reports.24 In addition, EDS pattern of PEGylated CsxWO3 nanorods (Fig. 3b) indicates that beside C and O element, there are Cs and W elements with the atomic ratio of Cs/W = 0.3, which is close to the typical hexagonal tungsten bronze structure of Cs0.32WO3. Based on XRD and EDS results, one can confirm the well formation of CsxWO3 sample.
Surface functional chemical groups of photothermal nanoagents are important for their biological applications.31 Herein, surface groups of CsxWO3 nanorods after PEGylation were identified by FT-IR spectrum (Fig. 4, blue curve). For comparison, the FT-IR spectrum of PEG-silane (black curve) was also shown in Fig. 4. Obviously, the FT-IR spectrum of PEGylated CsxWO3 nanorods is very similar to that of PEG-silane. Both the spectra exhibit a characteristic absorption band at 2960–2854 cm−1 and a peak at 1450 cm−1 corresponding to the C–H stretching modes and C–H deformation mode, respectively, of the –CH3 and –CH2 groups within the polymer chains.32 The band at 1350 cm−1 is corresponding to the polymer backbone.32 In addition, the band at 1099 cm−1 can be assigned to the vibration band of C–O–C.33 The only difference between the curves is high absorbance at 1000–700 cm−1 in the FT-IR spectrum of PEGylated CsxWO3 nanorods due to the NIR absorbance of CsxWO3 compound.34 Therefore, it can be deduced that there are PEG-silane on the surface of CsxWO3 nanorods after PEGylation.
In addition, we find that the as-prepared CsxWO3 nanorods before PEGylation could be dispersed in water under sonication, but could be precipitated in 3 hours. After PEGylation treatment, CsxWO3 nanorods could be readily dispersed in water, and the aqueous dispersion of PEGylated CsxWO3 nanorods (1.0 mg mL−1) exhibits a strong blue color (the inset of Fig. 5) and high stability, even remaining unchanged after a week due to the presence of PEG-silane. Subsequently, their optical property was investigated by UV-vis-NIR spectroscopy (Fig. 5). The spectrum is similar to what has been reported previously for the CsxWO3 nanomaterials16,24,28 and W18O49 nanowires.15,20 It exhibits a short-wavelength absorption edge at approximately 480 nm and reaches a minimum at around 570 nm. Importantly, CsxWO3 nanorods shows an increasing absorption with the increase of wavelength in the near-IR region (600–1100 nm), which origins from the free electrons and/or Cs-doping-induced small polarons.16 These facts confirm that PEGylated CsxWO3 nanorods have excellent hydrophilicity and strong NIR photoabsorption, and therefore have great potential as NIR photothermal nanoagents.
 | ||
| Fig. 5 UV-vis-NIR absorption spectrum of the PEGylated CsxWO3 aqueous dispersion with the concentration of 1.0 mg mL−1. The inset shows the PEGylated CsxWO3 aqueous dispersion. | ||
3.2 Comparison of 980 nm and 915 nm laser with CsxWO3 nanorods as photothermal nanoagents
Although the pure water is completely transparent to the naked eyes, it has a relatively strong optical absorption in the NIR range of 950–1050 nm (Fig. 6), similar to the previous report.35 For example, the optical absorption coefficient of pure water at 980 nm is about 0.216 cm−1 (Fig. 6), indicating that up to ∼40% of 980 nm light energy will be absorbed by water after propagating a distance of one centimeter in water according to the Beer–Lambert law. Thus, 980 nm laser light could not only serve as a heating source for heating water, but also has relatively low penetration depth in water. Since water is the most significant component of the animal and human body (60–70%),36 light around 980 nm is also capable of easily heating biological tissue specimens and has relatively low penetration depth in tissues. It should be noted that NIR laser at 980 nm is commonly used in NIR-PTA for cancer, due to the fact that the photothermal nanoagents can efficiently convert 980 nm light to heat energy.14,15,20,37 To avoid the overheating effect of NIR laser and improve its penetration depth in tissues, it may be a good choice to replace 980 nm laser with other NIR laser. Fortunately, semiconductor photothermal agents usually have an increasing absorption intensity with the wavelength in the near-IR region (600–1100 nm), as revealed in the photoabsorption spectrum (Fig. 5) of PEGylated CsxWO3 nanorods. Since CsxWO3 nanorods exhibit high absorption intensity at 915 nm (Fig. 5) and the water has very low absorption coefficient (about 0.030 cm−1) at 915 nm (Fig. 6), one can deduce that 915 nm laser may be a good alternative to 980 nm laser to overcome the water absorption and overheating effect.To experimentally investigate and compare the overheating effects of a 980 nm laser and a 915 nm laser, pure water or the PEGylated CsxWO3 aqueous dispersion were directly illuminated with a 980 or 915 nm laser with the same power density (0.72 W cm−2) in the absence of any barrier in laser path (Fig. 7a). When pure water was used as the model, the temperature elevation by 980 nm laser was 5 °C (Fig. 7b), which is 2.5 times as high as that (2 °C) by 915 nm laser, since the absorption coefficient of water at 980 nm is as high as 0.216 cm−1, ∼7 times higher than the value (0.030 cm−1) at 915 nm. Subsequently, when the PEGylated CsxWO3 dispersion (0.25 mg mL−1) was used as the model, the temperature elevation by 980 nm laser was 20.7 °C which is high compared with that (13.2 °C) by 915 nm laser (Fig. 7b). This fact should chiefly result from that CsxWO3 dispersion has high absorption coefficient at 980 nm (2.403), which is almost 1.2 times higher than the value (2.021) at 915 nm, as demonstrated in Fig. 5. Therefore, one can conclude that 980 nm laser as NIR light source has remarkable water overheating effect and high photothermal conversion performance, while 915 nm laser has a low overheating effect for water and also relatively low photothermal conversion performance.
For NIR-PTA of cancer, NIR laser should penetrate the skin and tissues to irradiate the photothermal nanoagents. To evaluate the effects of the skin and tissues on NIR laser, we coated the aqueous dispersion of PEGylated CsxWO3 nanorods (0.25 mg mL−1) with water or chicken skin as the barrier layer and model of biological tissue (Fig. 7c). Subsequently, 915 or 980 nm laser with the same power density (0.72 W cm−2) was used as NIR light source to penetrate through barrier layer and then irradiate the aqueous dispersion of PEGylated CsxWO3 nanorods, the temperature elevation of aqueous dispersion was recorded (Fig. 7d). With water (thickness: 10 mm) as the barrier layer, the temperature elevation by 980 nm laser decreases from 20.7 °C without water barrier layer to 13.2 °C with water barrier layer, indicating a remarkable reduction (7.5 °C, about 36%) due to strong optical absorption of water at 980 nm. In addition, the temperature elevation by 915 nm laser declines from 13.2 °C without water barrier layer to 10.9 °C with water barrier layer, suggesting a relatively small reduction (2.3 °C, about 17%) due to low optical absorption of water at 915 nm. Similarly, when chicken skin (thickness: ∼1 mm) was used as barrier layer, the temperature elevation by 980 nm laser decreases from 20.7 °C without any barrier layer to 12.4 °C with chicken skin layer, accompanying an obvious reduction (8.3 °C, about 40% decrease). This decrease should result from that chicken skin as barrier layer can absorb and/or scatter 980 nm laser, which is similar to our previous reports.2,38,39 On the contrary, the temperature elevation by 915 nm laser reduces from 13.2 °C without any barrier layer to 12.6 °C with chicken skin layer, revealing a neglectable decrease (0.6 °C, about 5% decrease) and then unobvious effect of chicken skin on 915 nm laser. More importantly, under the irradiation of 980 nm or 915 nm laser, the aqueous dispersion coated with water or skin exhibits very close temperature elevation (10.9–13.2 °C), indicating the similar photothermal conversion by 915/980 nm laser in vivo.
Based on the above results, one can conclude that compared with 980 nm laser as NIR light source, 915 nm laser exhibits a low overheating effect for water and also relatively low photothermal conversion ability in the absence of barrier layer, but has very close photothermal conversion ability in the presence of barrier layer (water or skin). Therefore, 915 nm laser can be considered to be a better NIR laser for NIR-PTA of cancer in vivo.
3.3 Photothermal performance and stability of CsxWO3 nanorods
Subsequently, the effects of the concentration of the PEGylated CsxWO3 nanorods on their photothermal performance were measured under the irradiation of 915 nm laser (0.72 W cm−2). Fig. 8 gives the temperature elevation of the aqueous dispersion (0.3 mL) of PEGylated CsxWO3 aqueous dispersion with various concentrations (0–1.0 mg mL−1). Pure water was used as a blank sample to compare with the PEGylated CsxWO3 nanorods. When water was irradiated by 915 nm laser with a power density of 0.72 W cm−2, the temperature of water showed unobvious increase. While the temperature of the PEGylated CsxWO3 aqueous dispersion (0.25–1.0 mg mL−1) went up sharply with the increase of the irradiated time to 30 s, and then exhibited a relatively flat upon further increase of the irradiated time to 500 s. The decline of heating up rate is due to faster heat loss at higher temperature.2,20 Moreover, the temperature change (ΔT) at 500 s (the inset of Fig. 8), which was calculated from the Fig. 8, went up with the increase of the PEGylated CsxWO3 nanorods concentration, from 16.9 °C for 0.25 mg mL−1, to 21.1 °C for 0.50 mg mL−1, 25.3 °C for 0.75 mg mL−1 and 27.6 °C for 1.00 mg mL−1. These results revealed that the PEGylated CsxWO3 nanorods can rapidly and efficiently convert the 915 nm laser energy into environmental heat.Furthermore, to investigate the long-term practical photothermal stability of these PEGylated CsxWO3 nanorods, the time-coursed temperature changes were tested. For comparison, PEGylated W18O49 nanowires were prepared and the long-term photothermal performance of PEGylated W18O49 nanowires was measured too. Both of the aqueous dispersions were prepared at the same concentration (1.0 mg mL−1) and stored in the plastic tubes with cap covered. The aqueous dispersions were irradiated one time per 24 h for 7 days, and the temperature changes of the aqueous dispersions were real-time recorded. As shown in Fig. 9, the temperature elevation change (ΔT) of the aqueous dispersion containing PEGylated W18O49 nanowires irradiated by 915 nm laser keeps at about 25.5 °C for the first 3 days, then starts to decrease to 23.3 °C at the fourth day and sharply declined to 1.5 °C (close to ΔT of pure water under the same condition) at the seventh day. While ΔT of PEGylated CsxWO3 nanorods kept steadily at about 27.4 °C under the same condition during 7 days. The long-term photothermal instability of the PEGylated W18O49 nanowires is due to the oxidization of the oxygen-deficiency-induced small polarons resulted from the multiple temperature increasement under O2 atmosphere results. A possible reason for the higher stability of CsxWO3 nanorods is that the polarons caused by the introduction of Cs+ are more stable than the oxygen vacancies in W18O49 nanowires. Therefore, the PEGylated CsxWO3 nanorods have a great potential to be used as a more efficient and stable 915 nm-laser-driven photothermal agent.
3.4 Cytotoxicity assay and PTA of cancer cells in vivo
The primary property of ideal photothermal coupling agents is nontoxic for biological applications.31,40 To evaluate the cytotoxicity of the PEGylated CsxWO3 nanorods, a MTT assay with the human cervical carcinoma cell line HeLa was used to determine their effect on cell proliferation. Fig. 10 presents cell viability values (%) versus incubation concentrations of the PEGylated CsxWO3 nanorods. When the concentration is not greater than 0.50 mg mL−1, the cellular viability is estimated to be greater than 90%. These data show that the PEGylated CsxWO3 aqueous dispersion (≤0.50 mg mL−1) can be considered to have low cytotoxicity. | ||
| Fig. 10 Cell viability values (%) of Hela cells incubated with the PEGylated CsxWO3 nanorods with different concentrations (0.25, 0.50, 0.75 mg mL−1) for 24 h, estimated by a MTT assay. | ||
It is well-known that hyperthermic oncology is the use of heat between 40 and 45 °C to damage cancer cells.15 The normal human body temperature is about 36 °C. After being injected with the PEGylated CsxWO3 aqueous dispersion, the tumor tissues can be easily heated to over 45 °C in 5 min under the irradiation of 915 nm laser with a power density of 0.72 W cm−2, probably resulting in the efficient death of tumor cells.15,20 In this study, PTA of tumor cells in mice was investigated by using the PEGylated CsxWO3 nanorods as photothermal nanoagents. For comparison, the effect of saline solution on PTA of tumor cells was also studied. Both the PEGylated CsxWO3 nanorods PBS dispersion and saline solution were introtumor injected to the SCID mice.
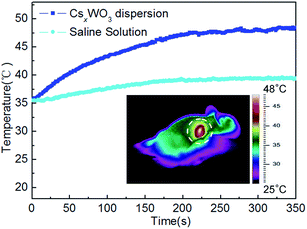
During the laser treatment, full-body thermographic images were captured by an infrared thermal imaging camera. For the mice injected with PEGylated CsxWO3 nanorods PBS dispersion (0.25 mg mL−1), the temperatures of the tumors were recorded as a function of the irradiation time, and a representative temperature plot was shown in Fig. 11. Obviously, the tumor surface temperature increased rapidly from 35.7 °C (body temperature) at 0 s to 40.9 °C at 60 s and 44.1 °C at 120 s, and then gradually elevated to a plateau of 48.2 ± 0.24 °C after 300 s. A representative thermographic image of nanorods treated mouse which was irradiated by laser at 300 s was also shown (the inset in Fig. 11). These facts reveal a rapid temperature elevation of the in vivo tumor, suggesting that the present PEGylated CsxWO3 nanorods within the tumor remained the excellent photothermal performance, which agrees well in the results showed by Fig. 7. On the contrary, for the mice injected with saline solution without PEGylated CsxWO3 nanorods, the elevation of tumor temperature is too low to damage tumor cells.
The histological examination of tumors was performed by the microscopic images (Fig. 12). For the SCID mice injected with saline, there was no obvious cell variation after the irradiation of 915 nm laser (Fig. 12a and b). This suggested that the very low heat-energy, which was converted from 915 nm laser by saline solution and/or tissues, had no influence on the cancer cells. On the contrary, for the PEGylated CsxWO3 nanorods-injected mice, significant transformations due to thermal cell necrosis (including cell shrinkage, nuclear damage, loss of contact, eosinophilic cytoplasm and etc.) were presented on most areas of the examined tumor slide (Fig. 12c and d). These facts suggested that in vivo cancer cells can be efficiently destroyed by the photothermal effect of the PEGylated CsxWO3 nanorods under 915 nm laser irradiation. Thus, the PEGylated CsxWO3 nanorods can be considered to be a promising candidate for PTA of in vivo tumor cells. In addition, the W-based semiconductor nanomaterials should be further developed to meet requests for future commercial use, for example, to develop multifunction by combining with other nanomaterials or medicines, and to further optimize the photothermal performances and biocompatibility of the W-based semiconductor nanomaterials.
4. Conclusion
The PEGylated CsxWO3 nanorods with an average particle size of ∼11 nm in diameter and ∼50 nm in length have been prepared by the solvothermal synthesis—PEGylation two-step route. These nanorods exhibit broad NIR absorption and high photothermal performance under the irradiation of 980/915 nm laser. 915 nm laser was experimentally proved to be a better light source than 980 nm laser due to its lower water overheat effect and better penetration. In addition, the PEGylated CsxWO3 nanorods exhibited more stable NIR photothermal performance than PEGylated W18O49 nanowires. Moreover, when the mice was injected with the PEGylated CsxWO3 nanorods, in vivo cancer cells can be efficiently destroyed by the photothermal effects of the PEGylated CsxWO3 nanorods, under the irradiation of 915 nm laser with the power density of 0.72 W cm−2 for 10 min. Therefore the 915 laser-driven PEGylated CsxWO3 nanorods have a potential as an efficient photothermal agent for NIR-induced PTA of cancer.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant no. 50925312, 51272299, 51273040, 51473033), Program for Changjiang Scholars and Innovative Research Team in University (Grant no. IRT1221, T2011079), High-Tech Research and Development Program of China (Grant no. 2012AA030309), the Scientific Research Foundation for the Returned Overseas Chinese Scholars, project of the Shanghai Committee of Science and Technology (13JC1400300), Innovation Program of Shanghai Municipal Education Commission (Grant no. 13ZZ053), Shanghai Leading Academic Discipline Project (Grant no. B603), the Program of Introducing Talents of Discipline to Universities (Grant no. 111-2-04), the Fundamental Research Funds for the Central Universities and DHU Distinguished Young Professor Program.References
- Z. Xiao, Nanomedicine, 2014, 9, 373–375 CrossRef CAS PubMed.
- Q. W. Tian, M. H. Tang, Y. G. Sun, R. J. Zou, Z. G. Chen, M. F. Zhu, S. P. Yang, J. L. Wang, J. H. Wang and J. Q. Hu, Adv. Mater., 2011, 23, 3542–3547 CrossRef CAS PubMed.
- Z. Zha, X. Yue, Q. Ren and Z. Dai, Adv. Mater., 2013, 25, 777–782 CrossRef CAS PubMed.
- Z. Zha, J. Wang, S. Zhang, S. Wang, E. Qu, Y. Zhang and Z. Dai, Biomaterials, 2014, 35, 287–293 CrossRef CAS PubMed.
- D. Wang, Z. Xu, H. Yu, X. Chen, B. Feng, Z. Cui, B. Lin, Q. Yin, Z. Zhang, C. Chen, J. Wang, W. Zhang and Y. Li, Biomaterials, 2014, 35, 8374–8384 CrossRef CAS PubMed.
- X. Li, M. Takashima, E. Yuba, A. Harada and K. Kono, Biomaterials, 2014, 35, 6576–6584 CrossRef CAS PubMed.
- Y. Wang, K. Wang, J. Zhao, X. Liu, J. Bu, X. Yan and R. Huang, J. Am. Chem. Soc., 2013, 135, 4799–4804 CrossRef CAS PubMed.
- J. Bai, Y. Liu and X. Jiang, Biomaterials, 2014, 35, 5805–5813 CrossRef CAS PubMed.
- Z. Zha, S. Wang, S. Zhang, E. Qu, H. Ke, J. Wang and Z. Dai, Nanoscale, 2013, 5, 3216–3225 RSC.
- S. S. Chou, B. Kaehr, J. Kim, B. M. Foley, M. De, P. E. Hopkins, J. Huang, C. J. Brinker and V. P. Dravid, Angew. Chem., Int. Ed., 2013, 52, 4160–4164 CrossRef CAS PubMed.
- C. M. Hessel, V. P. Pattani, M. Rasch, M. G. Panthani, B. Koo, J. W. Tunnell and B. A. Korgel, Nano Lett., 2011, 11, 2560–2566 CrossRef CAS PubMed.
- Q. W. Tian, F. R. Jiang, R. J. Zou, Q. Liu, Z. G. Chen, M. F. Zhu, S. P. Yang, J. L. Wang, J. H. Wang and J. Q. Hu, ACS Nano, 2011, 5, 9761–9771 CrossRef CAS PubMed.
- G. Song, Q. Wang, Y. Wang, G. Lv, C. Li, R. Zou, Z. Chen, Z. Qin, K. Huo, R. Hu and J. Hu, Adv. Funct. Mater., 2013, 23, 4281–4292 CrossRef CAS.
- Q. Tian, J. Hu, Y. Zhu, R. Zou, Z. Chen, S. Yang, R. Li, Q. Su, Y. Han and X. Liu, J. Am. Chem. Soc., 2013, 135, 8571–8577 CrossRef CAS PubMed.
- Z. G. Chen, Q. Wang, H. L. Wang, L. S. Zhang, G. S. Song, L. L. Song, J. Q. Hu, H. Z. Wang, J. S. Liu, M. F. Zhu and D. Y. Zhao, Adv. Mater., 2013, 25, 2095–2100 CrossRef CAS PubMed.
- Z. Zhou, B. Kong, C. Yu, X. Shi, M. Wang, W. Liu, Y. Sun, Y. Zhang, H. Yang and S. Yang, Sci. Rep., 2014, 4, 3653–3663 Search PubMed.
- L. Cheng, J. Liu, X. Gu, H. Gong, X. Shi, T. Liu, C. Wang, X. Wang, G. Liu, H. Xing, W. Bu, B. Sun and Z. Liu, Adv. Mater., 2014, 26, 1886–1893 CrossRef CAS PubMed.
- S. B. Lakshmanan, X. Zou, M. Hossu, L. Ma, C. Yang and W. Chen, J. Biomed. Nanotechnol., 2012, 8, 883–890 CrossRef CAS PubMed.
- Z. Liu, L. Cheng, L. Zhang, Z. Yang, Z. Liu and J. Fang, Biomaterials, 2014, 35, 4099–4107 CrossRef CAS PubMed.
- W. Xu, Q. Tian, Z. Chen, M. Xia, D. K. Macharia, B. Sun, L. Tian, Y. Wang and M. Zhu, J. Mater. Chem. B, 2014, 2(34), 5594–5601 RSC.
- F. Shi, J. Liu, X. Dong, Q. Xu, J. Luo and H. Ma, J. Mater. Sci. Technol., 2014, 30, 342–346 Search PubMed.
- G. Xi, S. Ouyang, P. Li, J. Ye, Q. Ma, N. Su, H. Bai and C. Wang, Angew. Chem., Int. Ed., 2012, 51, 2395–2399 CrossRef CAS PubMed.
- C. Guo, S. Yin, P. Zhang, M. Yan, K. Adachi, T. Chonan and T. Sato, J. Mater. Chem., 2010, 20, 8227–8229 RSC.
- C. Guo, S. Yin, L. Huang, L. Yang and T. Sato, Chem. Commun., 2011, 47, 8853–8855 RSC.
- J. Guo, C. Dong, L. Yang, G. Fu and H. Chen, Mater. Res. Bull., 2007, 42, 1384–1389 CrossRef CAS PubMed.
- C. Kasl and M. J. R. Hoch, J. Mater. Sci., 2013, 48, 3003–3012 CrossRef CAS PubMed.
- Z. Barkay, E. Grunbaum, G. Leitus and S. Reich, J. Supercond. Novel Magn., 2008, 21, 145–150 CrossRef CAS.
- C. Guo, S. Yin, H. Yu, S. Liu, Q. Dong, T. Goto, Z. Zhang, Y. Li and T. Sato, Nanoscale, 2013, 5, 6469–6478 RSC.
- C. Guo, S. Yin, P. Zhang, M. Yan, K. Adachi, T. Chonan and T. Sato, J. Mater. Chem., 2010, 20, 8227 RSC.
- B. Ingham, S. C. Hendy, S. V. Chong and J. L. Tallon, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 72, 075109 CrossRef.
- Z. Li, C. Wang, L. Cheng, H. Gong, S. Yin, Q. Gong, Y. Li and Z. Liu, Biomaterials, 2013, 34, 9160–9170 CrossRef CAS PubMed.
- G. Song, Q. Wang, Y. Wang, G. Lv, C. Li, R. Zou, Z. Chen, Z. Qin, K. Huo, R. Hu and J. Hu, Adv. Funct. Mater., 2013, 23, 4281–4292 CrossRef CAS.
- N. M. Logacheva, V. E. Baulin, A. Y. Tsivadze, E. N. Pyatova, I. S. Ivanova, Y. A. Velikodny and V. V. Chernyshev, Dalton Trans., 2009, 2482–2489 RSC.
- J. Liu, F. Shi, X. Dong, Q. Xu, S. Yin and T. Sato, Mater. Charact., 2013, 84, 182–187 CrossRef CAS PubMed.
- B. Li, Y. Zhang, R. Zou, Q. Wang, B. Zhang, L. An, F. Yin, Y. Hua and J. Hu, Dalton Trans., 2014, 43, 6244–6250 RSC.
- A. Bazzocchi, D. Diano, F. Ponti, A. Andreone, C. Sassi, U. Albisinni, G. Marchesini and G. Battista, Clin. Nutr., 2013, 32, 569–578 CrossRef PubMed.
- K. Dong, Z. Liu, Z. Li, J. Ren and X. Qu, Adv. Mater., 2013, 25, 4452–4458 CrossRef CAS PubMed.
- Z. Chen, L. Zhang, Y. Sun, J. Hu and D. Wang, Adv. Funct. Mater., 2009, 19, 3815–3820 CrossRef CAS.
- L. Zhang, Q. Tian, W. Xu, X. Kuang, J. Hu, M. Zhu, J. Liu and Z. Chen, J. Mater. Chem., 2012, 22, 18156–18163 RSC.
- Q. Chen, C. Wang, L. Cheng, W. He, Z. Cheng and Z. Liu, Biomaterials, 2014, 35, 2915–2923 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |