Surface-functionalized silica aerogels and alcogels for methylene blue adsorption†
Abstract
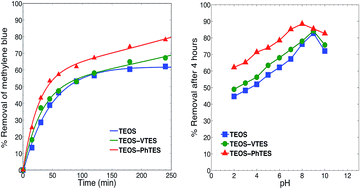
Surface-functionalized silica aerogels and alcogels prepared via a two-step sol–gel process through the combination of different silicon precursors were used in the adsorption of methylene blue dye molecules from aqueous media. The effect on the adsorption in batch reactors of the nature of precursors, the solvent used in the adsorbents synthesis, and the pH of the dye solution was monitored. Phenyl-functionalized silica materials revealed the highest adsorption capacity. Two phenyl-modified silica aerogels were widely tested in adsorption under various experimental conditions where the effect of pH, temperature, contact time, initial dye concentration, and adsorbent dose were investigated. The synthesis solvent was found to have a clear effect on the behavior of the adsorbent. Optimal conditions were found at pH 8 and 9 where the adsorbent–adsorbate surface charge interactions and the π–π stacking are most favourable. The adsorption followed a pseudo-second order kinetics, indicative of a co-existing chemisorption and physisorption processes. The adsorption data fitted the Sips isotherm and exhibited for the best aerogel a maximum adsorption capacity of 49.2 mg of dye per gram of adsorbent. The thermodynamic study revealed the adsorption of methylene blue onto phenyl-functionalized silica aerogels to be an exothermic and ordered adsorption process.

 Please wait while we load your content...
Please wait while we load your content...