Effect of the heat-induced whey proteins/κ-casein complex on the acid gelation of yak milk
Weiyi Xu,
Shenghua He*,
Ying Ma,
Yixin Zhang and
Rongchun Wang
School of Food Science and Engineering, Harbin Institute of Technology, Harbin 150090, China. E-mail: heshenghua@hit.edu.cn; Fax: +86 451 86282908; Tel: +86 451 86282908
First published on 22nd December 2014
Abstract
The effect of the heat-induced whey proteins/κ-casein complex on the acid gelation of yak milk was investigated. Both soluble whey protein/κ-casein complexes and the surface as micelle-bound complexes change the rheological properties of yoghurt. However, the soluble whey protein/κ-casein complexes were predominant in increasing the storage modulus (G′), water-holding capacity and firmness of acid gelation than micelle-bound complexes. The water-holding capacity and firmness increased with the increase of the amount of the soluble whey protein/κ-casein complexes. The water holding capacity and firmness of yoghurt from heated yak milk at pH 7.0 was higher than that at pH 6.6 and pH 7.4. The result also indicated that the amount of soluble heat-induced whey protein/κ-casein complexes were predominant in increasing the water holding capacity and firmness of yoghurt than the micelle-bound protein complexes.
Introduction
Heat treatment of milk is one of the important processes to either improve technological–functional properties or extend the shelf life of final products. Heat-treatment of milk can lead to the formation of heat-induced protein complexes between denatured whey proteins and casein. The heat-induced protein complexes are partly associated with casein micelles at the surface as micelle-bound complexes and partly in serum phase as soluble whey protein/κ-casein complexes.1 Most micelle-bound complexes occur at low pH and soluble whey proteins/κ-casein complexes occur at high pH after heat treatment.2 Therefore, pH is the key factor to determine the amount of the micelle-bound complexes and soluble whey proteins/κ-casein complexes during the heat treatment of milk. The formation of both heat-induced micelle-bound complexes and soluble whey proteins/κ-casein complexes affects the technological–functional properties of acid gelation. Yoghurt is a semi-solid food with a microstructure consisting of a protein network; the protein network is formed by the interaction of proteins.3,4The quality of yoghurt is determined by its microstructure and the rheological properties, which includes desirable functional and sensory properties such as smoothness, firmness and flow ability.5 Rheological properties and texture characteristics of yoghurt play a very important role in sensory evaluation and in consumer acceptability. In addition, the most typical defects of fermented milk products are low viscosity and reduced firmness, or these products have liquid consistency.6 These properties can be controlled by careful selection of the ingredients such as the milk source and concentration of milk solids, starter culture, additives and processing conditions. In yoghurt manufacture, milk was subjected to heat-treatment and resulting in the formation of heat-induced protein complexes. The formation of heat-induced whey proteins/κ-casein complexes can significantly increase the pH of acid gelation, and influences the firmness, water-holding capacity and cohesiveness.7 However, the mechanism of how the heat-induced whey proteins/κ-casein complexes affect the properties of acid gelation remains unclear.
The composition of yak milk is different from bovine milk, and yak milk contains higher content of total solid matter and protein than bovine milk; therefore, the yak milk is preferable to produce yoghurt.8 Otherwise, the content of β-lactoglobulin (β-Lg) and κ-casein (κ-CN) in yak milk is higher than bovine milk, and the amount of soluble whey proteins/κ-casein complexes is affected by the content of β-lactoglobulin (β-Lg) and κ-casein (κ-CN).9 Therefore, the amount of whey proteins/κ-casein complexes and the effect on acid gelation are different from bovine milk. In contrast to the detailed studies on bovine milk, there has been no information regarding the effect of the heat-induced whey proteins/κ-casein complex of yak milk on acid gelation. The aim of this study is to investigate the effect of heat-induced whey proteins/κ-casein complex on the properties of acid gelation of yak milk.
Materials and methods
Yak milk samples
Yak milk was sampled from Hongyuan county of Sichuan province in sterilized plastic containers and was chilled at −20 °C for the subsequent experiments.Heat treatment of the milk
The pH of the milk samples was adjusted to 6.6, 7.0 and 7.4 by the addition of 1 M of HCl and NaOH. The milk was then maintained at 4 °C. The milk was divided two parts, one part was not subjected to heat-treatment and pH adjustment as a control and the other was transferred to glass vials and immersed in a thermostatically controlled water-bath, the temperature of which was maintained at 85 ± 1 °C, and heated for 10 min with an additional 1 min rise-up time using an immersion circulator heater (Suzhou Bolinger Analytical Instruments Co., Ltd, China). After the heat treatment, the samples were immediately cooled to room temperature by immersing them in an ice bath.Preparation of the heat-induced aggregates and micellar casein
The serum phase of heat-treatment and unheated-treatment yak milk was separated by the ultracentrifugation of heated skimmed milk at 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g for 1 h at 4 °C in a Beckman L8-80M ultracentrifuge (Beckman Instruments Inc., Palo Alto, CA, USA). The clear serum was removed gently from each centrifuge tube with a syringe and the casein micelle was collected, and the clear serum and casein micelle separated from heat-treatment and the unheated-treatment yak milk was stored at 4 °C.
000 × g for 1 h at 4 °C in a Beckman L8-80M ultracentrifuge (Beckman Instruments Inc., Palo Alto, CA, USA). The clear serum was removed gently from each centrifuge tube with a syringe and the casein micelle was collected, and the clear serum and casein micelle separated from heat-treatment and the unheated-treatment yak milk was stored at 4 °C.
Mixture preparation
The serum phase of heated yak milk samples was mixed with casein micelle of unheated milk samples and gently stirred for 1 h, accordingly, the serum phase of unheated milk samples mixed with casein micelle of heated milk samples and gently stirred for 1 h. These two mixtures were adjusted to the same content of total solid matter.Acid gelation behaviour of milk samples
The mixtures were equilibrated at 37 °C prior to acid gelation by the addition of 17 g glucono-δ-lactone (GDL) in 1 kg of mixtures. The formation of the gels was monitored using an AR2000 rheometer (TA Instruments, Guyancourt, France) equipped with a cone-plane geometry and using the oscillatory mode at a frequency of 1 Hz and a strain of 0.1%. The gelation time was defined as the moment when G′ > 1 Pa.Preparation of yoghurt
Three yoghurts were thus prepared: (a) control yoghurt, produced with unheated yak milk; (b) yoghurt produced with a mixture of milk, the serum phase from heated yak milk samples blended with casein micelle from unheated yak milk samples (SHUHC) and (c) yoghurt produced with a mixture of milk, the serum phase from unheated yak milk samples blended with casein micelle from heated milk samples (SUHHC). Three different milk samples were preheated to 37 °C, and a starter culture of Meihua Biotechnology (Shanghai, China) containing L. delbrueckii subsp. Bulgaricus, S. thermophilus, B. longum subsp. Infantis and B. infantis were inoculated into the milk mixture, each at a ratio of 1% (w/w). The inoculated yoghurt mix was mixed thoroughly and poured in 150 mL polystyrene cups with lids; these were incubated at 37 °C until the pH reached 4.5.Four yoghurts were thus prepared: (a) control yoghurt, produced with unheated yak milk; (b) pH 6.6, yak milk was adjusted to 6.6 by the addition of 1 M of HCl and adjusted to pH 6.65 after heat treatment. (c) pH 7.0, yak milk was adjusted to 7.0 by the addition of 1 M of NaOH and adjusted to pH 6.65 after heat treatment at 85 ± 1 °C for 10 min. (d) pH 7.4, yak milk was adjusted to 7.4 by the addition of 1 M of NaOH and adjusted to pH 6.65 after heat treatment. Yoghurt was prepared according to the abovementioned method.
Water-holding capacity
The water-holding capacity (WHC) of the fermented samples was measured by centrifugation at 5000 × g using a centrifuge. Samples (25 g) were weighed into a falcon plastic tube, capped and centrifuged for 25 min at 4 °C (Sodini, Mattas, & Tong, 2006). The whey (top layer) was carefully removed and weighed. The WHC was carried out in duplicate and expressed as a percentage using the following equation:| WHC (%) = [(sample weight − expelled whey weight)/sample weight] × 100 |
Measurement of firmness of yoghurt
A TA-XT 2 texture analyser (Stable Micro Systems, UK) with a P25 Perspex cylindrical probe (diameter 25 mm) and 25 kg load cell was used. All measurements were carried out at 20 °C. The peak force and cohesiveness (g) required to break the gel was used to measure the firmness of yoghurt. The depth of penetration was 20 mm while the speed of penetration was 1 mm s−1.Statistical analysis
Data were analyzed by the ANOVA method using SPSS software, version 10.0 (SPSS, Inc., Chicago, IL, USA). The differences among the means of the analysis data were compared at a significance level of p < 0.05.Results and discussion
Rheological properties of acid gelations and yoghurts
Understanding of the rheological properties of yoghurt is important in process design, product development, processing, handling, quality control and storage.10 The G′ value is used as a measure of the deformation energy stored in the sample during the shear process and it represents the elastic behaviour of the sample.11 Fig. 1 shows the storage modulus (G′) of control acid gelation, SHUHC acid gelation and SUHHC acid gelation obtained using a frequency sweep from 0.1 to 10 Hz. The storage modulus (G′) of SHUHC and SUHHC acid gelation is higher than the control acid gelation. These results indicate that both micelle-bound complexes and soluble whey protein/κ-casein complexes change the rheological properties of yoghurt. The storage modulus (G′) of SHUHC acid gelation increased quicker than the SUHHC acid gelation, and this result indicates that soluble whey protein/κ-casein complexes were predominant in changing the rheological properties of yoghurt than micelle-bound complexes. The higher values of the storage modulus (G′) of SHUHC and SUHHC acid gelation compared to the control acid gelation indicated interactions between the whey proteins and the casein formed network of the acid gelation. This may be due to electrostatic interactions of the caseins with whey proteins. These interactions can strengthen the gel structure leading to higher elastic characteristics of the acid gelation with higher G′ values. The increase of G′ may be due to the increased proportion of covalent disulphide bonds in the heat-induced whey protein/κ-casein complexes. Vasbinder et al. reported that thiol/disulphide exchanges continue throughout acidification and significantly contribute to the final gel strength.12The G′ increased for SHUHC and SUHHC acid gelation; however, this was particularly prominent for the control yoghurt. This was most likely due to protein rearrangement during heating, leading to higher protein–protein interactions, as suggested by Doleyres et al.13 This rearrangement produced a yoghurt structure with a more solid-like nature.
The gelation time was defined as the moment when G′ > 1 Pa. The gel time of the control, SHUHC and SUHHC acid gelation is shown in Fig. 2. The gel time of SHUHC and SUHHC acid gelation is significantly shorter than the control acid gelation. This result indicates that both micelle-bound complexes and soluble whey protein/κ-casein complexes in heated yak milk can decrease the acid gel time compared to unheated yak milk. This result agreed with a previous report which stated that soluble and micelle-bound whey protein/κ-casein complexes of bovine milk can take responsibility for the early gelation point.7
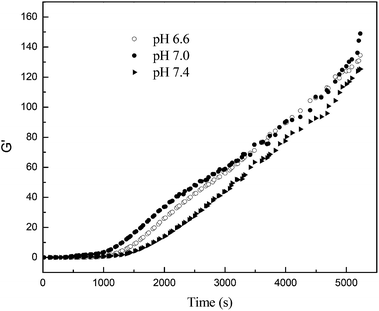
Fig. 3 shows the storage modulus (G′) of yak milk heated at pH 6.6, 7.0 and 7.4. Yak milk heated at pH 6.6, 7.0 and 7.4 showed that the storage modulus (G′) was not significantly different. Although the amount of soluble whey protein/κ-casein complexes in heated yak milk increased with the increase of pH from 6.6 to 7.4, both the micelle-bound and soluble whey protein/κ-casein complexes in heated yak milk can increase the G′; therefore, there were no significant difference in the storage modulus for heated yak milk at different pH.
The water holding capacity of yoghurts
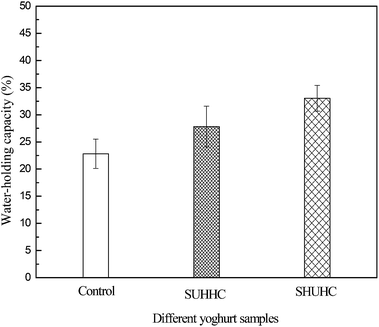
The water holding capacity of control yoghurt, SHUHC and SUHHC yoghurts is presented in Fig. 4. The water holding capacity of SHUHC and SUHHC yoghurts was higher than the control yoghurt, and the water holding capacity of SHUHC yoghurt is higher than SUHHC yoghurts. This result may be due to the whey protein denaturation and the formation of heat-induced complexes creating new porous structures, into which water influxes and immobilises.14,15 The heat-induced whey protein/κ-casein complexes can promote the formation of homogenous porosity. Therefore, the acid gels can hold a lot of water in the protein gel network. The water holding capacity of yak milk heated at pH 6.6, 7.0 and 7.4 is presented in Fig. 5. The result shows that the water holding capacity of yoghurt from heated yak milk at pH 6.6, 7.0 and 7.4 was higher than the control yoghurt, and the water holding capacity of yoghurt from heated yak milk at pH 7.0 was higher than that at pH 6.6 and pH 7.4. The result indicated that the amount of soluble heat-induced whey protein/κ-casein complexes were predominant in increasing the water holding capacity of yoghurt than the micelle-bound protein complexes. Otherwise, the high water holding capacity of yoghurt from heated yak milk at pH 7.0 compared to pH 6.6 and pH 7.4 may be due to the water holding capacity of yoghurt or may be due to the amount of soluble whey protein/κ-casein complexes because the amount of soluble whey protein/κ-casein complexes was highest in serum when yak milk was heated at pH 7.0 compared to pH 6.6 and pH 7.4. Li et al. reported that the amount of soluble whey protein/κ-casein complexes was highest in serum when yak milk was heated at pH 7.0 than at pH 6.6 and pH 7.4.16The firmness of yoghurts
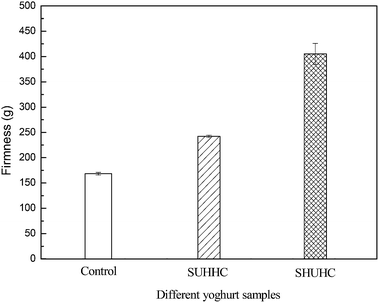
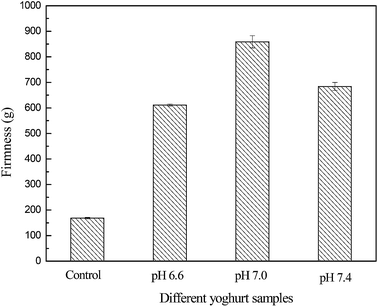
Firmness is considered as one of the most important parameters for the textural perception of yoghurts.17 The firmness of the control yoghurt, SHUHC and SUHHC yoghurts is presented in Fig. 6. The firmness of SHUHC and SUHHC yoghurts was significantly higher than the control yoghurt. The increase of firmness in SHUHC and SUHHC yoghurts may be due to denatured whey protein aggregated by a heat-induced effect with casein and κ-casein and formed the micelle-bound complexes and soluble whey protein/κ-casein complexes, respectively. These two complexes can increase the firmness of yoghurt probably due to protein aggregation that forms a protein network. Otherwise, the firmness of the SHUHC yoghurts was significantly higher than the SUHHC yoghurt. The content of the soluble whey protein/κ-casein complexes in the SHUHC yoghurts was higher than in the SUHHC yoghurt. However, the content of the micelle-bound complexes in SUHHC yoghurt was higher than in SHUHC yoghurts, this result indicates that the soluble whey protein/κ-casein complexes was predominant in strengthening of the acid gel network than the micelle-bound complexes. Guyomarc'h et al. reported that the whey protein/κ-casein complexes may be important to either the onset of acid gelation or the strengthening of the acid gel network.1The firmness of yoghurt from heated yak milk at pH 6.6, pH 7.0 and pH 7.4 is shown in Fig. 7. The firmness of yoghurt from heated yak milk at pH 6.6, pH 7.0 and pH 7.4 was significantly higher than the control yoghurt. The firmness of yoghurt from heated yak milk at pH 7.0 was significantly higher than at pH 6.6 and pH 7.4. This result may be because the amount of soluble whey protein/κ-casein complexes of yoghurt from heated yak milk at pH 7.0 was higher than pH 6.6 and pH 7.4 or may be the soluble whey protein/κ-casein complexes was predominant in increasing the firmness of yoghurt than the micelle-bound complexes.14 The high content of soluble whey protein/κ-casein complexes of yoghurt from heated yak milk can strength the protein network and increase the firmness of yoghurt. Otherwise, the firmness of yak milk yoghurt is significantly higher than bovine due to yak milk containing higher content of total solid matter and protein.9,18 The firmness of yoghurt from heated yak milk at pH 6.6, pH 7.0 and pH 7.4 was significantly higher than SHUHC and SUHHC yoghurts due to heated yak milk at pH 6.6, pH 7.0 and pH 7.4 consisting of the micelle-bound complexes and soluble whey proteins/κ-casein complexes.
Conclusion
In this study, we found that both soluble whey protein/κ-casein complexes and the surface as micelle-bound complexes change the rheological properties of acid gelation. However, the soluble whey protein/κ-casein complexes were predominant in improving the technological–functional properties of yoghurt than micelle-bound complexes.Acknowledgements
This work was supported by China Postdoctoral Science Foundation Grant (2012M520756; 2014T70360), from Chinese Postdoctoral Science Foundation Commission. The Fundamental Research Funds for the Central Universities (Grants no. HIT. NSRIF. 2014094).References
- F. Guyomarc'h, A. J. R. Law and D. G. Dalgleish, J. Agric. Food Chem., 2003, 51, 4652 CrossRef PubMed.
- L. Donato and D. G. Dalgleish, J. Agric. Food Chem., 2006, 54, 7804 CrossRef CAS PubMed.
- J. A. Lucey, P. A. Munro and H. Singh, J. Food Sci., 1998, 63, 660 CrossRef CAS PubMed.
- I. Sodini, F. Remeuf, S. Haddad and G. Corrieu, Crit. Rev. Food Sci., 2004, 44, 113 CrossRef PubMed.
- W. J. Lee and J. A. Lucey, J. Texture Stud., 2003, 34, 515 CrossRef PubMed.
- A. Y. Tamime and R. K. Robinson, Yoghurt: Science and technology, Woodhead Publishing, Cambridge, UK, 3rd edn, 2007, ch. 2 and 5 Search PubMed.
- M. Morand, F. Guyomarc'h and M. H. Famelart, Dairy Sci. Technol., 2011, 91, 97 CrossRef CAS.
- S. H. He, Y. Ma, Q. M. Li, J. Q. Wang, X. Yang, S. H. Tang and H. M. Li, J. Food Comp. Anal., 2011, 24, 223 CrossRef CAS PubMed.
- H. M. Li, Y. Ma, A. J. Dong, J. Q. Wang, Q. M. Li, S. H. He and J. L. Maubois, Dairy Sci. Technol., 2010, 90, 111 CrossRef CAS PubMed.
- J. F. Velez-Ruiz, Rheological properties of yogurt, in Progress in food engineering research and development, ed. J. M. Cantor, Nova Science Publishers Inc, New York, 2008, pp. 223–242 Search PubMed.
- A. G. Cruz, R. N. Cavalcanti, L. M. R. Guerreiro, A. S. Sant'Ana, L. C. Nogueira, C. A. F. Oliveira, R. Deliza, R. L. Cunha, J. A. F. Faria and H. M. A. Bolini, J. Food Eng., 2013, 114, 323 CrossRef CAS PubMed.
- A. J. Vasbinder, A. C. Alting, R. W. Visschers and C. G. de Kruif, Int. Dairy J., 2003, 13, 29 CrossRef CAS.
- Y. Doleyres, L. Schaub and C. Lacroix, J. Dairy Sci., 2005, 88, 4146 CrossRef CAS.
- V. P. Denisov, B. H. Jonsson and B. Halle, Nat. Struct. Biol., 1999, 6, 253 CrossRef CAS PubMed.
- R. Hinrichs, J. Gotz, M. Noll, A. Wolfschoon, H. Eibel and H. Weisser, Int. Dairy J., 2004, 14, 817 CrossRef CAS PubMed.
- Q. M. Li, Y. Ma, S. H. He, E. Walid, W. Y. Xu, J. Q. Wang and L. Y. Qiu, Int. Dairy J., 2014, 35, 102 CrossRef CAS PubMed.
- F. Harte, S. Clark and G. V. Barbosa-Cánovas, J. Food Eng., 2007, 80, 990 CrossRef PubMed.
- P. H. P. Prasanna, A. S. Grandison and D. Charalampopoulos, Food Res. Int., 2013, 51, 15 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |