Biotemplated synthesis of hierarchically nanostructured TiO2 using cellulose and its applications in photocatalysis†
Abstract
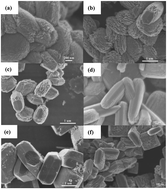
Hierarchically nanostructured TiO2 has been hydrothermally synthesized using cellulose as a biotemplate involving various types of acids. We show that the surface charges of nanocrystalline cellulose and reaction parameters including reaction temperature, acid to cellulose ratio and reaction time have a large effect on the morphology and nanostructures of TiO2 products. The photocatalytic activity of as-synthesized, calcined hierarchically nanostructured TiO2 and calcined hierarchically nanostructured TiO2 loaded Au nanoparticles is evaluated by photo-degradation of methyl orange under white light.

 Please wait while we load your content...
Please wait while we load your content...