Base-mediated direct fluoroalkenylation of 2-phenyl-1,3,4-oxadiazole, benzothiazole and benzoxazole with gem-difluoroalkenes†
Xuxue Zhang,
Yingyin Lin,
Juan Zhang and
Song Cao*
Shanghai Key Laboratory of Chemical Biology, School of Pharmacy, East China University of Science and Technology (ECUST), Shanghai 200237, China. E-mail: scao@ecust.edu.cn; Fax: +86-21-64252603; Tel: +86-21-64253452
First published on 22nd December 2014
Abstract
Direct α-fluorovinylation of 2-phenyl-1,3,4-oxadiazole, benzothiazole and benzoxazole with gem-difluoroalkenes via nucleophilic vinylic substitution reaction (SNV) under the assistance of KHMDS or NaH at room temperature is described.
The alkenylated heteroarenes have gained much attention over the past decades due to their widespread applications as versatile building blocks for the synthesis of pharmaceuticals, agrochemicals and functional materials.1 As a consequence, considerable efforts have been made to develop efficient methods for the synthesis of alkenyl-substituted heteroarenes.2 The traditional method to prepare the alkenyl-substituted heteroarene is the Knoevenagel-type condensation of 2-methyl heteroarene with the appropriate aldehyde.3
Nowadays, the direct conversion of C–H bonds into C–C bonds is a very useful methodology for the construction of complex molecules.4 At the same time, direct C–H alkenylation through C–H bond cleavage has been developed into a powerful tool for the synthesis of alkenylated arenes and particularly alkenylated heteroaromatics.5 Among the protocols for the direct C–H alkenylation of (hetero)arenes, the alkenylation of (hetero)arenes with alkenyl halides by using palladium or copper as catalyst is becoming more popular.6
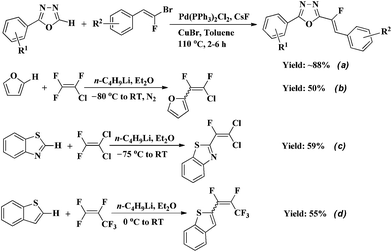
Monofluoroalkenes are useful fluorinated synthons in synthetic organic chemistry and precursors of biologically active compounds.7 In medicinal chemistry and peptide chemistry, they are often considered as peptide bond isosteres.8 However, very few reports are available on the synthesis of fluoroalkenyl-substituted heterocycles. In 2013, Schneider et al. reported a novel and facile approach to heteroarylated monofluoroalkenes through the base-assisted Pd- and Cu-catalyzed direct C–H alkenylation of various 1,3,4-diazoles using gem-bromofluoroalkenes as electrophiles (Scheme 1a).9
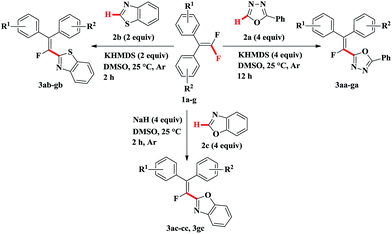
Generally, alkenyl chlorides, bromides or iodides could be served as alkenylating agents in the coupling with heteroarenes.10 However, unreactive alkenyl fluorides are rarely used as coupling partners or substrates in alkenylation reaction due to the unique nature of the carbon–fluorine bond.11 Up to now, there are only three examples of direct C–H alkenylation of heterocycles with fluoroalkenes (Scheme 1b–d).12 The drawbacks of these methods are the lithiation of heterocycles with the moisture sensitive n-butyllithium in the first step, very low reaction temperature, relatively low yield, the mixture of E/Z isomers and the need for highly electron-deficient polyfluoroalkenes. Furthermore, n-butyllithium is prone to nucleophilic attack of fluorine atom in fluoroalkene, followed by fluorine elimination to produce undesired product.12a Thus, a simple method for the direct C–H alkenylation of heterocycles with fluoroalkenes under mild conditions is highly desirable. In continuation of our research on the functionalization of gem-difluoroalkenes,13 in this communication, we report a straightforward protocol for direct C–H bond monofluoroalkenylation of 2-phenyl-1,3,4-oxadiazole, benzothiazole and benzoxazole with gem-difluoroalkenes under the assistance of base (KHMDS or NaH) at room temperature (Scheme 2).
We began our investigation by using (2,2-difluoroethene-1,1-diyl)dibenzene 1a and 2-phenyl-1,3,4-oxadiazole 2a as the model substrates to optimize the reaction conditions and the results are summarized in Table 1. Initially, the effect of base on the reaction was examined. In the absence of the base, the reaction hardly proceeded and no expected product 3aa was observed (entry 1). Among the various bases evaluated, KHMDS was better than the other bases such as t-BuOLi, n-BuLi, KOH, NaOH, LiHMDS and NaHMDS, and could provide the highest yield (entries 2–10). Although KOH and NaOH are cheaper and commercially more readily available than KHMDS, the lower yields of the valuable desired products render them less economically attractive. Too much or too little KHMDS led to a remarkable decrease in the yield (entries 11–12). Decreasing the amount of 2a resulted in low yield of 3aa (entries 13–14). The results also indicated that the solvent has a dramatic effect on the reaction. Only DMSO could afford the expected product 3aa in good yield (entries 10, 15–19). Moreover, increasing reaction temperature obviously diminished the yield (entries 20–21). Finally, we also used the reaction system of literatures (n-BuLi/Et2O, entry 22), however, no desired product was detected.
| Entry | 2a (equiv.) | Base (equiv.) | Temp (°C) | Solvent | Yield of 3aab (%) |
|---|---|---|---|---|---|
| a Reagents and conditions: 1a (1.0 mmol), KHMDS (4.0 mmol, 1.0 mol L−1 in THF), DMSO (5 mL), 12 h.b Yields were determined by GC-MS analysis and based on 1a. | |||||
| 1 | 4 | — | 25 | DMSO | 0 |
| 2 | 4 | LiOH (4) | 25 | DMSO | 20 |
| 3 | 4 | t-BuOK (4) | 25 | DMSO | 27 |
| 4 | 4 | t-BuOLi (4) | 25 | DMSO | 64 |
| 5 | 4 | n-BuLi (4) | 25 | DMSO | 68 |
| 6 | 4 | KOH (4) | 25 | DMSO | 70 |
| 7 | 4 | NaOH (4) | 25 | DMSO | 71 |
| 8 | 4 | LiHMDS (4) | 25 | DMSO | 55 |
| 9 | 4 | NaHMDS (4) | 25 | DMSO | 50 |
| 10 | 4 | KHMDS (4) | 25 | DMSO | 81 |
| 11 | 4 | KHMDS (5) | 25 | DMSO | 26 |
| 12 | 4 | KHMDS (3) | 25 | DMSO | 17 |
| 13 | 3 | KHMDS (4) | 25 | DMSO | 23 |
| 14 | 2 | KHMDS (4) | 25 | DMSO | 16 |
| 15 | 4 | KHMDS (4) | 25 | DMF | 17 |
| 16 | 4 | KHMDS (4) | 25 | DMA | 8 |
| 17 | 4 | KHMDS (4) | 25 | THF | 0 |
| 18 | 4 | KHMDS (4) | 25 | Dioxane | Trace |
| 19 | 4 | KHMDS (4) | 25 | Et2O | 0 |
| 20 | 4 | KHMDS (4) | 70 | DMSO | 22 |
| 21 | 4 | KHMDS (4) | 40 | DMSO | 21 |
| 22 | 4 | n-BuLi (4) | 25 | Et2O | 0 |
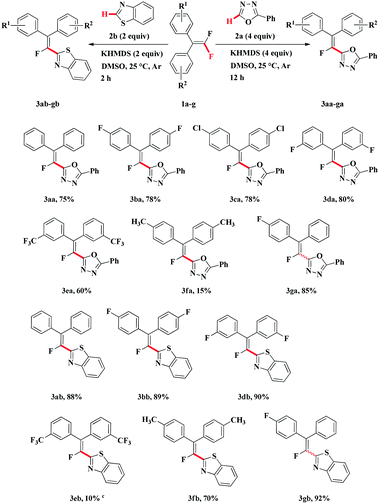
With the optimized reaction conditions established (Table 1, entry 10), we proceeded to investigate the direct C–H alkenylation of 2-phenyl-1,3,4-oxadiazole 2a with several gem-difluoroalkenes (Table 2, 3aa–ga). The results indicated that gem-difluoroalkenes bearing electron-withdrawing groups on the benzene ring were somewhat more reactive and provided the alkenylated 1,3,4-oxadiazoles in good yields (3ba–da). However, the gem-difluoroalkenes bearing strong electron-withdrawing group such as CF3 was unfavorable for the reaction (3ea). gem-Difluoroalkene having electron-donating group such as CH3 was not a suitable substrate for this transformation and furnished only small amount of alkenylated product (3fa). Unsymmetrical gem-difluoroalkene could also furnish high yield of desired product (3ga), but with poor E/Z selectivity (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, the ratio of E/Z isomers in the crude reaction mixture was determined by 19F NMR). When 1-(2,2-difluorovinyl)-4-methoxybenzene was used as substrate, no reaction was observed. It appears that two diaryl groups attached at the C–C double bond of gem-difluoroalkenes is necessary, which could stabilize carbanion transition state. Furthermore, the replacement of phenyl group in 2-phenyl-1,3,4-oxadiazole with hydrogen or alkyl group can not make the reaction proceed smoothly.
3, the ratio of E/Z isomers in the crude reaction mixture was determined by 19F NMR). When 1-(2,2-difluorovinyl)-4-methoxybenzene was used as substrate, no reaction was observed. It appears that two diaryl groups attached at the C–C double bond of gem-difluoroalkenes is necessary, which could stabilize carbanion transition state. Furthermore, the replacement of phenyl group in 2-phenyl-1,3,4-oxadiazole with hydrogen or alkyl group can not make the reaction proceed smoothly.
To explore the scope of the novel alkenylation reaction, we next evaluated the reaction of benzothiazole 2b with different gem-difluoroalkenes (Table 2, 3ab–gb). Much to our delight, 2.0 equiv. KHMDS and 2.0 equiv. benzothiazole 2b were enough to make the reaction proceed smoothly. Furthermore, gem-difluoroalkenes were almost completely consumed within 2 hours, affording the desired products in high yield (3ab, 3bb, 3db, 3fb). This might be in part due to the ease of dissociation of the C–H bond in benzothiazole 2b in the presence of base. Contrary to the reactions of 2-phenyl-1,3,4-oxadiazole 2a, the reaction of benzothiazole 2b with gem-difluoroalkene bearing electron-donating group such as CH3 could give good yield of alkenylated product (3fb), whereas gem-difluoroalkene bearing CF3 resulted in very low conversion (3eb). Unsymmetrical gem-difluoroalkene could react with benzothiazole 2b smoothly and the expected product (3gb) was isolated in excellent yield but in a poor stereoselectivity. It should be noted that when NaOH or KOH were used as base, which were suitable for the reaction of gem-difluoroalkenes with 2-phenyl-1,3,4-oxadiazole 2a, the reaction could not proceed efficiently.
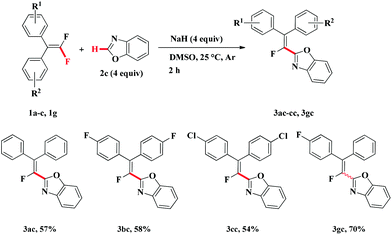
Under the above-mentioned two optimized conditions, the scope of the direct C–H alkenylation reaction involving C–F bond breaking was expanded to benzoxazole 2c (Table 3). However, KHMDS as well as other bases such as t-BuOLi, t-BuOK, KOH and LiHMDS failed to furnish the desired products. Gratifyingly, we found that the alkenylation of benzoxazole 2c with gem-difluoroalkenes 1a–c, 1g could occur in presence of NaH and give the expected alkenylated products in moderate yield at room temperature (3ac–cc, 3gc). Unfortunately, both compound 1f having electron-donating group and compound 1e bearing strong electron-withdrawing group on benzene ring were not suitable substrates for the transformation and only trace amounts of alkenylated products were detected.
Based on the above observations, we suggest that the mechanism is analogous to those proposed in the literatures.14 gem-Difluoroalkenes undergo nucleophilic vinylic substitution (SNV) with heteroarenes in the presence of base via addition–elimination processes to afford fluoroalkenylated heteroaromatics. The abstraction of the C–H proton of heteroaromatics with assistance of base to generate the corresponding sp2 carbanion on the heterocycles is essential for efficient transformation. The presence of benzene group in 2-phenyl-1,3,4-oxadiazole, benzothiazole and benzoxazole is favorable for the activation of the C–H bond.
In conclusion, we have developed a mild and efficient method for the KHMDS or NaH-mediated alkenylation of three azole heterocycles, including 2-phenyl-1,3,4-oxadiazole, benzothiazole and benzoxazole with various gem-difluoroalkenes. Although, at present stage, the substrate scope of this new approach was relatively limited due to the fact that the cleavage of heteroaromatic C–H bonds is much more difficult in absence of metal catalyst, it will pave the way for realizing direct C–H alkenylation of heterocycles without prefunctionalization of Csp2–H of heterocycles such as halogenation. Further studies to expand the substrates and increase yields are in progress in our laboratory.
Acknowledgements
We are grateful for financial supports from the National Natural Science Foundation of China (Grant nos 21472043, 21272070), and the Key Project in the National Science & Technology Pillar Program of China in the twelfth five-year plan period (2011BAE06B01-15).Notes and references
- (a) S. M. Rida, F. A. Ashour, S. A. M. El-Hawash, M. M. ElSemary, M. H. Badr and M. A. Shalaby, Eur. J. Med. Chem., 2005, 40, 949 CrossRef CAS PubMed; (b) F.-S. Chang, W. Chen, C. Wang, C.-C. Tzeng and Y.-L. Chen, Bioorg. Med. Chem., 2010, 18, 124 CrossRef CAS PubMed; (c) G. S. He, L.-S. Tan, Q. Zheng and P. N. Prasad, Chem. Rev., 2008, 108, 1245 CrossRef CAS PubMed; (d) Y.-J. Sun, N. Li, Z.-B. Zheng, L. Liu, Y.-B. Yu, Z.-H. Qin and B. Fu, Adv. Synth. Catal., 2009, 351, 3113 CrossRef CAS; (e) J. Vinsova, K. Cermakova, A. Tomeckova, M. Ceckova, J. Jampilek, P. Cermak, J. Kunes, M. Dolezal and F. Staud, Bioorg. Med. Chem., 2006, 14, 5850 CrossRef CAS PubMed; (f) A. Fournet, R. Mahieux, M. A. Fakhfakh, X. Franck, R. Hocquemiller and B. Figadère, Bioorg. Med. Chem. Lett., 2003, 13, 891 CrossRef CAS; (g) L. Meng, Y. Kamada, K. Muto, J. Yamaguchi and K. Itami, Angew. Chem., Int. Ed., 2013, 52, 10048 CrossRef CAS PubMed.
- (a) A. L. Gottumukkala, F. Derridj, S. Djebbar and H. Doucet, Tetrahedron Lett., 2008, 49, 2926 CrossRef CAS PubMed; (b) F. Besselièvre, S. Lebrequier, F. Mahuteau-Betzer and S. Piguel, Synthesis, 2009, 3511 Search PubMed; (c) F. Besselièvre, S. Piguel, F. Mahuteau-Betzer and D. S. Grierson, Org. Lett., 2008, 10, 4029 CrossRef PubMed; (d) C. Verrier, C. Hoarau and F. Marsais, Org. Biomol. Chem., 2009, 7, 647 RSC; (e) B. Qian, P. Xie, Y. Xie and H. Huang, Org. Lett., 2011, 13, 2580 CrossRef CAS PubMed; (f) W.-C. Lee, T.-H. Wang and T.-G. Ong, Chem. Commun., 2014, 50, 3671 RSC; (g) G. C. Senadi, W.-P. Hu, J.-S. Hsiao, J. K. Vandavasi, C.-Y. Chen and J.-J. Wang, Org. Lett., 2012, 14, 4478 CrossRef CAS PubMed; (h) A. Nakhi, S. Archana, G. P. Kumar Seerapu, K. S. Chennubhotla, K. L. Kumar, P. Kulkarni, D. Haldar and M. Pal, Chem. Commun., 2013, 49, 6268 RSC.
- (a) P. J. Sanfilippo, M. Urbanski, J. B. Press, Z. G. Hajos, D. A. Shriver and C. K. Scott, J. Med. Chem., 1988, 31, 1778 CrossRef CAS; (b) A. Beutler, C. D. Davies, M. C. Elliott, N. M. Galea, M. S. Long, D. J. Willock and J. L. Wood, Eur. J. Org. Chem., 2005, 3791 CrossRef CAS; (c) I. N. Houpis, A. Molina, J. Lynch, R. A. Reamer, R. P. Volante and P. J. Reider, J. Org. Chem., 1993, 58, 3176 CrossRef CAS; (d) C. Compton and W. Bergmann, J. Org. Chem., 1947, 363 CrossRef CAS; (e) O. Cox, H. Jackson, V. A. Vargas, A. Báez, J. I. Colón, B. C. González and M. León, J. Med. Chem., 1982, 25, 1378 CrossRef CAS.
- (a) D. R. Stuart and K. Fagnou, Science, 2007, 316, 1172 CrossRef CAS PubMed; (b) D. A. Colby, R. G. Bergman and J. A. Ellman, Chem. Rev., 2010, 110, 624 CrossRef CAS PubMed; (c) C. Li, P. Li, J. Yang and L. Wang, Chem. Commun., 2012, 48, 4214 RSC; (d) T. He, L. Yu, L. Zhang, L. Wang and M. Wang, Org. Lett., 2011, 13, 5016 CrossRef CAS PubMed; (e) Z. Mao, Z. Wang, Z. Xu, F. Huang, Z. Yu and R. Wang, Org. Lett., 2012, 14, 3854 CrossRef CAS PubMed; (f) X.-W. Liu, J.-L. Shi, J.-X. Yan, J.-B. Wei, K. Peng, L. Dai, C.-G. Li, B.-Q. Wang and Z.-J. Shi, Org. Lett., 2013, 15, 5774 CrossRef CAS PubMed.
- (a) S. Cui, L. Wojtas and J. C. Antilla, Org. Lett., 2011, 13, 5040 CrossRef CAS PubMed; (b) R. Gao and C. S. Yi, J. Org. Chem., 2010, 75, 3144 CrossRef CAS PubMed; (c) D. J. Schipper, M. Hutchinson and K. Fagnou, J. Am. Chem. Soc., 2010, 132, 6910 CrossRef CAS PubMed; (d) A. García-Rubia, R. G. Arrayás and J. C. Carretero, Angew. Chem., Int. Ed., 2009, 48, 6511 CrossRef PubMed.
- (a) B. P. Berciano, S. Lebrequier, F. Besselièvre and S. Piguel, Org. Lett., 2010, 12, 4038 CrossRef PubMed; (b) Z.-J. Wang, J.-G. Yang, F. Yang and W. Bao, Org. Lett., 2010, 12, 3034 CrossRef CAS PubMed; (c) F. Besselièvre, S. Piguel, F. Mahuteau-Betzer and D. S. Grierson, Org. Lett., 2008, 10, 4029 CrossRef PubMed; (d) D. I. Chai and M. Lautens, J. Org. Chem., 2009, 74, 3054 CrossRef CAS PubMed; (e) B. Das, G. C. Reddy, P. Balasubramanyam and N. Salvanna, Tetrahedron, 2012, 68, 300 CrossRef CAS PubMed.
- (a) S. Eddarir, Z. Abdelhadi and C. Rolando, Tetrahedron Lett., 2001, 42, 9127 CrossRef CAS; (b) K. R. Karukurichi, R. Salud-Bea, W. J. Jahng and D. B. Berkowitz, J. Am. Chem. Soc., 2007, 129, 258 CrossRef CAS PubMed; (c) S. S. F. Leung, J. Tirado-Rives and W. L. Jorgensen, Bioorg. Med. Chem. Lett., 2009, 19, 1236 CrossRef CAS PubMed; (d) C.-S. Yu, L.-W. Chiang, C.-H. Wu, Z.-K. Hsu, M.-H. Lee, S.-D. Pan and K. Pei, Synthesis, 2006, 3835 CrossRef CAS PubMed; (e) Y. Asahina, K. Iwase, F. Iinuma, M. Hosaka and T. Ishizaki, J. Med. Chem., 2005, 48, 3194 CrossRef CAS PubMed; (f) B. Malo-Forest, G. Landelle, J.-A. Roy, J. Lacroix, R. C. Gaudreault and J.-F. Paquin, Bioorg. Med. Chem. Lett., 2013, 23, 1712 CrossRef CAS PubMed.
- (a) S. D. Edmondson, L. Wei, J. Xu, J. Shang, S. Xu, J. Pang, A. Chaudhary, D. C. Dean, H. He, B. Leiting, K. A. Lyons, R. A. Patel, S. B. Patel, G. Scapin, J. K. Wu, M. G. Beconi, N. A. Thornberry and A. E. Weber, Bioorg. Med. Chem. Lett., 2008, 18, 2409 CrossRef CAS PubMed; (b) S. Oishi, H. Kamitani, Y. Kodera, K. Watanabe, K. Kobayashi, T. Narumi, K. Tomita, H. Ohno, T. Naito, E. Kodama, M. Matsuoka and N. Fujii, Org. Biomol. Chem., 2009, 7, 2872 RSC; (c) S. Couve-Bonnaire, D. Cahard and X. Pannecoucke, Org. Biomol. Chem., 2007, 5, 1151 RSC; (d) Y. Asahina, K. Iwase, F. Iinuma, M. Hosaka and T. Ishizaki, J. Med. Chem., 2005, 48, 3194 CrossRef CAS PubMed; (e) S. Osada, S. Sano, M. Ueyama, Y. Chuman, H. Kodama and K. Sakaguchi, Bioorg. Med. Chem., 2010, 18, 605 CrossRef CAS PubMed; (f) P. V. Veken, K. Senten, I. Kertèsz, I. D. Meester, A.-M. Lambeir, M.-B. Maes, S. Scharpé, A. Haemers and K. Augustyns, J. Med. Chem., 2005, 48, 1768 CrossRef PubMed.
- C. Schneider, D. Masi, S. Couve-Bonnaire, X. Pannecoucke and C. Hoarau, Angew. Chem., Int. Ed., 2013, 52, 3246 CrossRef CAS PubMed.
- (a) J. J. Mousseau, J. A. Bull and A. B. Charette, Angew. Chem., Int. Ed., 2010, 49, 1115 CrossRef CAS PubMed; (b) S. Sahnoun, S. Messaoudi, J.-D. Brion and M. Alami, Eur. J. Org. Chem., 2010, 6097 CrossRef CAS; (c) F. Besselièvre, S. Lebrequier, F. Mahuteau-Betzer and S. Piguel, Synthesis, 2009, 3511 Search PubMed; (d) A. L. Gottumukkala, F. Derridj, S. Djebbar and H. Doucet, Tetrahedron Lett., 2008, 49, 2926 CrossRef CAS PubMed.
- (a) H. Amii and K. Uneyama, Chem. Rev., 2009, 109, 2119 CrossRef CAS PubMed; (b) J. L. Kiplinger, T. G. Richmond and C. E. Osterberg, Chem. Rev., 1994, 94, 373 CrossRef CAS.
- (a) S. Dixon, J. Org. Chem., 1956, 400 CrossRef CAS; (b) K. Okuhara, J. Org. Chem., 1976, 41, 1487 CrossRef CAS; (c) R. P. Gajewski, J. L. Jackson, N. D. Jones, J. K. Swartzendruber and J. B. Deeter, J. Org. Chem., 1989, 54, 3311 CrossRef CAS.
- (a) Y. Xiong, X.-X. Zhang, T. Huang and S. Cao, J. Org. Chem., 2014, 79, 6395 CrossRef CAS PubMed; (b) W.-P. Dai, J. Xiao, G.-Y. Jin, J.-J. Wu and S. Cao, J. Org. Chem., 2014, 79, 10537 CrossRef CAS PubMed; (c) G. Jin, J. Zhang, W. Wu and S. Cao, J. Fluorine Chem., 2014, 168, 240 CrossRef CAS PubMed.
- J. Ichikawa, Y. Wada, M. Fujiwara and K. Sakoda, Synthesis, 2002, 1917 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: General procedure for synthesis, characterization data, and 1H, 13C, 19F NMR and HRMS spectra of compounds 3. See DOI: 10.1039/c4ra13761b |
| This journal is © The Royal Society of Chemistry 2015 |