Enantioselective assembly of functionalized carbocyclic spirooxindoles using an l-proline derived thiourea organocatalyst†
Abstract
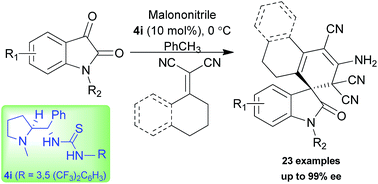
Sequential vinylogous Michael addition–cyclization reactions of vinyl malononitriles with isatylidene malononitrile were accomplished using L-proline derived bifunctional thiourea. Cyclohexylidine malononitrile afforded exclusively the single diastereomer with good to excellent enantioselectivity (up to 99% ee) for diverse oxindole spirocyclohexene derivatives. Tetralone derived α,α-dicyano alkene was also employed to access spirocyclic oxindole scaffolds with an excellent level of stereoselectivity (up to 99% ee).

 Please wait while we load your content...
Please wait while we load your content...