One-pot synthesis of Pd@PdPt core–shell nanocubes on carbon supports
Abstract
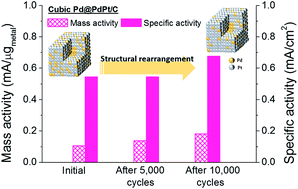
Cubic Pd@PdPt core–shell nanoparticles were synthesized on carbon supports using a one-pot process. The as-prepared cubic Pd@PdPt/C exhibited high electrocatalytic activity for oxygen reduction reactions. The enhancement in the mass activity increased after accelerated durability tests due to structural rearrangement.

 Please wait while we load your content...
Please wait while we load your content...