A photocatalytic process for the eradication of dengue through ˙OH generation in the presence of sunlight and iron oxide†
G. V. Pereira,
V. A. Freitas,
H. S. Oliveira,
L. C. A. Oliveira* and
J. C. Belchior
Department of Chemistry, Federal University of Minas Gerais, BH-MG, Av. Antônio Carlos 6627, Campus Pampulha, 31270-901-Brazil. E-mail: luizoliveira@qui.ufmg.br; jadson@ufmg.br; Fax: +55 31 3409 5700; Tel: +55 31 3409 7550
First published on 19th November 2014
Abstract
Iron oxide was dispersed in a floating matrix based on autoclaved porous brick. Under an incidence of solar radiation ˙OH radicals are generated by the photocatalytic process, completely oxidizing the organic matter. This procedure makes the proliferation of dengue larva unfeasible. Additionally, ˙OH radicals have a remarkable deleterious effect on hatching of A aegypti eggs. The predominant iron phase was hematite (α-Fe2O3), as indicated by the Mossbauer spectroscopy. The formation of ˙OH species was demonstrated by typical m/z values obtained via ESI-MS.
Dengue is a disease that occurs mainly in tropical and subtropical areas of the world. It is caused by an arbovirus producing infected individuals that can develop a high fever, headaches, pain behind the eyes and back, drowsiness and generalized body ache, nausea and vomiting. Reddish spots may appear throughout the body.1,2 The disease transmission occurs in all cases through the bite of the Aedes aegypti mosquito. This species reached South America via slave ships from Africa in the colonial period (16th to 19th centuries).3,4 Dengue is a particularly serious concern to public health in several tropical countries.5 From 2009 to 2011, the Department of Health Surveillance of the Ministry of Health in Brazil reported incidences of 205.5, 530.3 and 400.5 per 100
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 persons, respectively.6 Globally, about 100 million cases of dengue and about 500
000 persons, respectively.6 Globally, about 100 million cases of dengue and about 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cases of dengue hemorrhagic fever occur annually.7,8 Therefore, efficient technological processes than can contribute to reduce the incidence of dengue in infected areas are highly needed.
000 cases of dengue hemorrhagic fever occur annually.7,8 Therefore, efficient technological processes than can contribute to reduce the incidence of dengue in infected areas are highly needed.
The present study discloses a new use of a catalyst comprising iron oxide supported on an array of autoclaved aerated concrete. The density, porosity and mechanical strength of the support material make it well suited to be used in the degradation process of organic compounds. This support material can be chemically treated with Fe2O3, which is a low cost material. The use of this material can aid in avoiding the proliferation of diseases based vectors, whose initial stage of the development occurs in aquatic environments.9,10 We report the use of catalysts consisting of iron oxide supported by an autoclaved aerated concrete array. This material is activated by UV component of sunlight and can be used for degrading microorganisms, such as bacteria, fungi and plankton that serve as a basis for feeding the larvae of A aegypti that can cause diseases such as dengue, or other vectors that can produce diseases such as yellow fever, malaria, and leishmaniasis. In other words, the present approach can, in principle, eliminates larvae that are developed in an aqueous medium.
The catalyst in contact with an aqueous environment under incidence of sunlight or artificial light in the ultraviolet range produces the hydroxyl radical (˙OH) with a standard oxidation potential of 2.8 eV.11,12 This potential energy is the highest potential available from a natural source. In a sense, the device described herein is similar to a Noxer block, in which a porous cement matrix is adsorbed with TiO2 and then calcinated.13,14 In addition, this powerful reactant is transformed into water after the process, characterizing our approach as a green method and an in nature process. The (˙OH) radical degrades organic compounds, including the larvae food source or even the larvae themselves, reaching complete degradation of the organic matter.
In the synthesis of the α-Fe2O3 catalyst, 12 or 25 g of autoclaved aerated concrete was used, treated in 100 mL of a solution of iron(III) nitrate concentration of 1 mol L−1 for 30 min. The material was thermally treated at 200 °C for 2 h and called PT. The analogue material, without chemical modification, was called PNT. Scanning electron microscopy (SEM/EDS) was carried out on a JEOL analyzer. 57Fe Mössbauer spectra of all samples were collected in a constant acceleration transmission mode with a ∼50 mCi 57Co/Rh source. A spectrometer equipped with a transducer (CMTE model MA250) controlled by a linear function-driving unit (CMTE model MR351) was used to obtain the spectra at 298 K. The data were stored in a 1024 channel-MCS memory unit, with the Doppler velocity ranging between ±10 mm s−1, calibrated with a metallic iron (α-Fe) foil as absorber. The absorbers were prepared with a uniform thickness of ∼10 mg Fe per cm2, admixing sucrose to the samples. The experimental resonance lines were fitted to Lorentzian functions with the least-square fitting statistical procedure of the NORMOSTM-90 computer program. UV-vis spectroscopy with diffuse reflectance geometry was carried out in a Shimadzu 2600 spectrometer from 200 to 800 nm. BaSO4 powder was used as reference material (100% reflectance), and the Kubelka–Munk equation was used to manipulate all data. The actual chemical compositions of the prepared samples were determined with an inductively Coupled Plasma Mass Spectrometry (ICP-MS) ELAN DRC II (PerkinElmer Life and Analytical Sciences, USA). The ICP-MS was operated with Pt sampler and skimmer cones, both purchased from Perkin Elmer. The plates containing the Aedes aegypti eggs were submerged in water. After 24 h, the hatching occurred and the catalysts were added into the medium. The beakers containing the larvae and the catalysts were placed under solar irradiation (average temperature = 20 °C, humidity = 69% and radiation kJ m2 = 2000) for 8 hours (http://www.inmet.gov.br/).
The efficiency of the catalyst material was compared with the oxidative activity of commercial sodium hypochlorite (bleach), which is one of the most commonly compounds used in the eradication of insect larvae. The experiments were divided as follows: (i) sucrose (200 mg L−1) was used as nutrient solution; (ii) sucrose solution + bleach; (iii) sucrose solution + 12 g of catalyst and (iv) sucrose solution + 25 g of catalyst. Each type of sample was placed in different natural conditions for 8 weeks, namely: outdoor shade, shade with protection from rainfall and direct exposure to sunlight. The experiments were monitored for 1 week with the following experimental conditions: average temperature = 20 °C, humidity = 69% and radiation = 2000 kJ m2 (http://www.inmet.gov.br/).
The formation of iron(III) impregnated in the autoclaved aerated concrete matrix oxide (PT) was characterized by 57Fe Mössbauer spectroscopy at 20 K (spectrum not shown). The PNT sample has four iron species before impregnation and heat treatment: Fe3+, α-Fe2O3, Fe7S8 and α-Fe, with the most abundant species being the superparamagnetic Fe3+ followed by α-Fe2O3 (26%). After impregnation and heat treating (200 °C/2 h) the sulfide species are converted into hematite (α-Fe2O3), by aerobic oxidation of sulfide to SO2(g). The material continues to show the superparamagnetic Fe3+ as majority iron specie. This is probably due to the reduced size of iron oxide particles (<10 nm) dispersed onto aerated concrete matrix resulting from impregnation.
The determination of total iron in the PNT sample by an Inductively Coupled Plasma Mass Spectrometry (ICP-MS) presented a content of 1.9 and 3.5% (w/w) before and after impregnation, respectively. Through SEM-EDS it is possible to observe that the impregnation method allows spreading α-Fe2O3 homogeneously on the whole surface (Fig. 1a–c). The PT sample shows a smooth surface at 50 μm compared to the roughness of the PNT surface (Fig. 1d–f). It is also observed and demonstrated that the iron species dispersion on the PNT sample is heterogeneous by collecting the EDS signals from different regions of the sample (spectra not shown here). In the PNT central region, the SKα signal from Fe7S8 appears while the FeKα signal from other iron species is very low. By observing the borderline direction, the FeKα becomes stronger. This suggests that the iron present in the parent material comes from its manufacture. However, the amount and chemical speciation of iron atoms present in the matrix do not provide the efficiency required to perform our new approach. Actually, only the impregnation process may provide iron photocatalytically activity that can be used for the catalytic process.
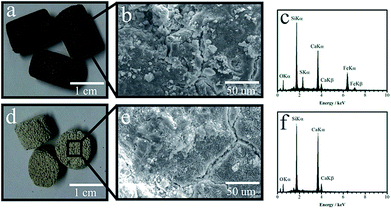 | ||
| Fig. 1 Photograph of the PT sample (a), SEM image of its surface (b) and the EDS spectra of the area (c). PNT sample (d), SEM image of its surface (e) and the EDS spectra of the area (f). | ||
Based on the spectral relative area of the iron species, it is observed that about 40% of the iron content initially lies in the form of iron sulfide and metallic iron. Another important feature is that after the impregnation process, the iron species are well distributed on the whole surface of the support material. On the other hand, the pre-existing iron is homogeneously-mixed in autoclaved aerated concrete and hence it does not participate at all in the catalytic process. The UV-vis diffuse reflectance spectra of the as-prepared materials are shown in Fig. S1.† The PNT spectrum showed no absorption in the spectral region surrounding regions of the visible and ultraviolet (red line in Fig. S1†). The spectrum of the PT material (black line), presented absorption in the ultraviolet region and visible region between 400–600 nm, whereas the solar radiation that reaches the earth surface comprises part of the region where the iron oxide absorbs (highlighted in the graphic part), which means that this catalyst can be excited by solar radiation. Accordingly, the catalyst should exhibit photocatalytic properties for the generation of electron pair (e−)/holes (h+) when irradiated with sunlight capable of generating radical species. In order to prove the formation of hydroxyl radicals (highly oxidizing species), experiments with an organic dye (methylene blue) were performed via injection. Some authors (Guimarães et al. (2008)15 and Oliveira et al. (2007))16 have demonstrated that the presence of hydroxyl radicals is evidenced by the formation of species with m/z = 300, 301, 316 and 318, which are related to the incorporation of the radical into the structure. Hydroxylated species with m/z = 302, 318 and 329 were seen after 30 min under UV light irradiation (Fig. 2). After 120 min, the molecular ion of the dye (m/z = 284) decreased sharply relative to other hydroxylated species. Likewise, the PNT material showed hydroxylated species after 30 min of irradiation. However, the presence of these species was much less pronounced when compared to the PT material, and after 120 min. the signal intensity did not significantly change (Fig. 2). Therefore, the chemical and thermal treatment on PNT material is actually essential to increase photoactivity and generate the radical species.17
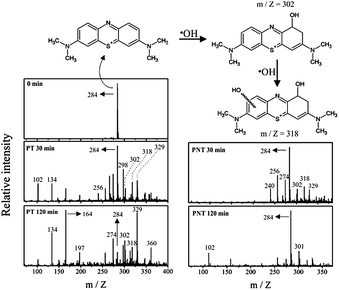 | ||
| Fig. 2 Generation of radical species through catalysts under UV-light irradiation followed by ESI-MS spectroscopy on positive mode. | ||
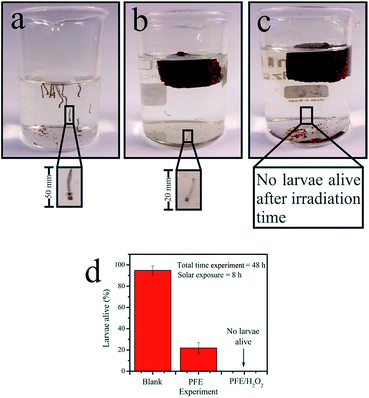
As a preliminary analysis, we studied the ability of the PT material to eliminate dengue larvae at an advanced stage of its development. Fig. 3a shows the exposure to 8 h under sunlight radiation. The larvae did not change after this long period of exposition and hence it seems that there was no effect at all. Similarly, Fig. 3b illustrates the results at the same condition but in the presence the PT material. As one can see there are no visible changes for the larvae. Fig. 3c shows an experiment similar to Fig. 3b but using an artificial 15 W UV (λ = 285 nm) light source. For these particular results (Fig. 3b and c) one can verify that due to the radiation and catalytic effects the larvae did not grow significantly. Furthermore, we observed that under sunlight only 20% of larvae survived. However, a very efficient result was verified considering artificial UV light that produced a significant result, 100% of larvae died.
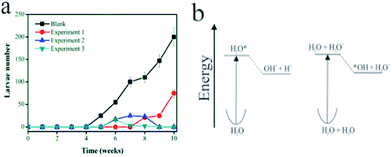
In order to validate the general applicability of the catalyst in the control of mosquito larvae, we carried out field experiments simulating natural conditions. These experiments were performed in four different sites (occupied houses) with weekly data collection. The materials were tested during 10 weeks under solar radiation. The photodegradation of a nutrient solution composed of sucrose (200 mg L−1) was studied through the PT material to demonstrate the potential application for mosquito larvae eradication. The nutrient solution was exposed for 24 h in a batch reactor under sunlight irradiation with, as well as without, catalyst. After 24 h, the solutions were analyzed through total organic carbon (TOC), total carbon (TC) and inorganic carbon (IC). The experiments were carried out in triplicate in order to evaluate the statistical analysis. It is clearly seen that after 24 h the material provided efficient activity capable of degrading 90% of all organic matter (Fig. 4a). This makes the aqueous environment a nutrient-scarce medium for the development of mosquito larvae, leaving, on average, 10% of organic matter initially present. Certainly, a longer time exposed to this process will eventually mineralize all organic materials in solution.
In order to determine the efficiency of mosquito larvae eradication, catalyst material was subjected to a preliminary test. The latter involves the exposition of the photocatalytic material in different aqueous environments allowing the proliferation of disease vectors such as dengue, malaria, and yellow fever. Fig. 4a shows the evolution of weekly average number of larvae developed for each type of solution. The result demonstrates the efficiency of the catalyst system in inhibiting the proliferation of insect larvae, probably due to the consumption of organic matter in the aqueous medium. The sample with only sucrose solution showed the highest number of dengue larvae (see black dots) while no larvae were observed in the sample containing bleach solution besides sucrose before 7 weeks. Between 7 and 9 weeks, some larvae developed (Fig. 4a), presumably due to a lower light incidence or the elapsed presence of rain in this period. However, it is well known that bleach solution is not environmentally friendly. In addition, its use can be diluted, in principle, during rainy seasons or by any other water supply. Accordingly, in the following weeks (until the time analyzed) one observes a loss of efficiency of the bleach action process due to the appearance of about 100 larvae. Also, bleach – comprised of hypochlorite (ClO−) and often NaOH as a stabilizing agent – is photolabile and decomposes relatively fast upon exposure to sunlight for 8 h and soon losing its oxidative ability. This result can, in principle, address a useful advantage of our photocatalytic system. In the case of the proposed approach using α-Fe2O3 dispersed on the support material, one can observe that a total elimination of larvae occurred in both quantities namely 12 and 25 g employed in the support. Clearly, for both concentrations (blue and green symbols) total elimination of larvae was observed, demonstrating a quite efficient method for eradicating Aedes A aegypti and avoiding possible proliferation.
The general mechanism of this photocatalysis11 requires the semiconductor excitation with light, followed by the formation of an electron–hole pair on the surface of the catalyst. The hydroxyl radical ˙OH is formed by the photocatalytic decomposition of water in the presence of the activating Fe2O3 matrix or by the reaction with the anion –OH. The radical ˙OH is extremely strong and non-selective oxidant, which partially or completely decomposes various organic molecules and this characteristic can be exploited to aid in the elimination of vectors such as those of dengue, malaria, yellow fever, West Nile fever, filariasis, and mosquito-borne encephalitis as well as chikungunya antilles.
The highly oxidizing radical may act on the eradication of dengue by three main mechanisms: (i) promoting the oxidation of organic matter decreasing food for the larvae; (ii) reacting directly with the larvae or mosquito eggs, attacking cell walls of these species or even (iii) reducing the possibility of larvae to access oxygen at the water surface. The ionization of water leads to its decomposition into OH radicals, since the H2O+ cation formed at the Frank–Condon geometry is unstable (Fig. 4b). On the other hand, the potential energy of the OH species shows a minimum both in the anion and radical forms and does not decompose. Water theoretical decomposition studies have illustrated the isolate molecule in the gas phase. From the theoretical point of view, it is interesting to note that an isolated water molecule would not decompose,18 but rather dissociates to OH+(X3Σ) + H(2S). The formation of an H+ ion is only stable in the presence of another water molecule to accept it.
The catalyst was also effective in preventing the eggs from hatching. In the presence of the dengue larvae (provided by the local Brazilian government) and sunlight, but in the absence of catalyst the experiments show, as expected, the generation of dengue larvae (see the arrows in Fig. 4a). On the other hand, in the presence of the catalyst under sunlight (Fig. 4b) there was no dengue larvae growth. These results show that the proposed process completely inhibits the formation of the larvae due to the in situ generation of hydroxyl radicals through a photocatalytic process. As inferred from Mössbauer spectroscopy, the iron species are highly dispersed on the surface of the porous support matrix resulting in very small sized particles. Moreover, one should consider the fact that the materials have a lower density than water, the materials are floating, which means that the light absorption is maximized facilitating the photocatalytic process. Evidence of radical action was seen through optical microscopy in Fig. S2.†
For the present study live larvae (left column in Fig. S2†) and larvae killed by the action of the catalyst under sunlight (the two columns to the right in Fig. S2†) were chosen. The images show that for live larvae, not very obvious spots are observed. On the other hand, in the images of dead larvae, sharp spots are easily seen (indicated by arrows), which may be attributed to reactions with radicals generated by the photocatalytic process. Nene et al. 2007 (ref. 7) pointed out that mosquito management is currently the only prevention option for incidence of dengue. Such an approach may not provide an efficient method to eradicate it. However, our process may contribute to better solve this larvae proliferation that produces the Aedes aegypti. Actually, it provides a methodology that causes the death of larvae in all their stages of development before becoming a mosquito. Actually, the process described herein parallels one of Mother Nature's means to control mosquito populations, since the action of ˙OH radicals on the decreasing of larvae proliferation is known and occurs naturally in the Rio Negro, which is an Amazon River tributary in northern Brazil. It has been shown18 that a large amount of Chromobacterium Violaceum bacteria (Bacillus violaceus) has photobiological action, generating free radicals that prevent the proliferation of not only dengue larvae, but those of yellow fever and malaria. Thus, although the dengue incidence is high in north of Brazil (Amazon), the region around the Rio Negro does not present a high incidence of the disease. In this work we imitate nature, which promotes a controlled and efficient way to generate ˙OH radicals,19 which preclude A aegypti eggs from hatching and also kill the mosquito's larvae (Fig. 5).
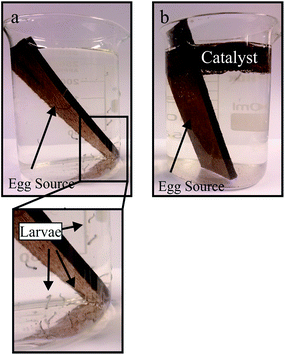 | ||
| Fig. 5 Hatching tests. (a) Tests under sunlight in the absence of PT material. (b) The presence of PT. | ||
Conclusions
In summary, here we have presented a very effective procedure to prevent the hatching of dengue larvae and also to kill larvae already hatched. The generation of hydroxyl radicals by the iron oxide-based catalyst deposited over a floating autoclaved brick act on dengue larvae. Similarly, it also prevents the eggs from hatching by direct attack of the radical ˙OH. A major advantage of the present approach is the generation of this radical by excitation of the iron catalyst by solar radiation and this photocatalytic reaction has the particular advantage of occurring at the water surface. This makes the whole process a low cost procedure and hence can be widely used by the population with a capacity for a large-scale application of it.Acknowledgements
This work was supported by the CNPq, FAPEMIG, CAPES and PRPq (UFMG). Vértica Serviços e Tecnologia Eireli.Notes and references
- M. D. R. Q. Lima, R. M. R. Nogueira, A. M. B. Filippis, P. C. G. Nunes, C. S. Souza and M. H. F. B. Silva, J. Virol. Methods, 2014, 204, 105 CrossRef CAS PubMed.
- C. B. Ocampo, N. J. Mina, M. Carabalí, N. Alexander and L. Osorio, Acta Trop., 2014, 132, 15 CrossRef PubMed.
- B. Jain, U. C. Chaturvedi and A. Jain, Microb. Pathog., 2014, 69, 45 CrossRef PubMed.
- R. C. Leite, A. I. Souza, P. M. S. Castanha, M. T. Cordeiro, C. T. Martelli, G. Ferreira, L. Katz and C. Braga, J. Clin. Virol., 2014, 60, 16 CrossRef CAS PubMed.
- V. Duong, C. Simmons, L. Gavotte, A. Viari, S. Ong, N. Chantha, N. J. Lennon, B. W. Birren, S. Vong, J. J. Farrar, M. R. Henn, V. Deubel, R. Frutos and P. Buchy, Infect., Genet. Evol., 2013, 15, 59 CrossRef CAS PubMed.
- F. O. Ferraz, M. R. Q. Bomfim, A. H. Totola, T. V. Avila, D. Cisalpino, J. E. M. Pessanha, D. G. Souza, A. L. Teieira, M. L. Nogueira, O. B. Romero and M. M. Teixeira, J. Clin. Virol., 2013, 58, 41 CrossRef PubMed.
- V. Nene and J. R. Wortman, Science, 2007, 316, 1718 CrossRef CAS PubMed.
- S. Bhatt, P. W. Gething, O. J. Brady, J. P. Messina, A. W. Farlow, C. L. Moyes, J. M. Drake, J. S. Brownstein, A. G. Hoen, O. Sankoh, M. F. Myers, D. B. George, T. Jaenisch, G. R. W. Wint, C. P. Simmons, T. W. Scott, J. J. Farrar and S. I. Hay, Nature, 2013, 496, 504 CrossRef CAS PubMed.
- J. V. Coelho, M. S. Guedes, R. G. Prado, J. Tronto, J. D. Ardisson, M. C. Pereira and L. C. A. Oliveira, Appl. Catal., B, 2014, 144, 792 CrossRef CAS PubMed.
- Y. Xia, H. Daí, H. Jiang, L. Zhang, J. Deng and J. Liu, J. Hazard. Mater., 2011, 186, 84 CrossRef CAS PubMed.
- W. F. de Souza, I. R. Guimarães, M. C. Guerreiro and L. C. A. Oliveira, Appl. Catal., A, 2009, 360, 205 CrossRef CAS PubMed.
- M. C. Pereira, F. S. Coelho, C. C. Nascentes, J. D. Fabris, M. H. Araujo, K. Sapag, L. C. A. Oliveira and R. M. Lago, Chemosphere, 2010, 81, 7 CrossRef CAS PubMed.
- Y. Feng, D. W. Smith and J. R. Bolton, J. Environ. Eng. Sci., 2007, 6, 277 CrossRef CAS.
- S. Makins, Chem. Rev., 2001, 11, 22–23 Search PubMed.
- I. R. Guimarães, L. C. A. Oliveira, P. F. Queiroz, T. C. Ramalho, M. Pereira, J. D. Fabris and J. D. Ardisson, Appl. Catal., A, 2008, 347, 89 CrossRef PubMed.
- L. C. A. Oliveira, T. C. Ramalho, M. Gonçalves, F. Cereda, K. T. Carvalho, M. S. Nazzarro and K. Sapag, Chem. Phys. Lett., 2007, 446, 133 CrossRef CAS PubMed.
- M. González, M. Gilibert, A. Aguilar and R. Sayós, J. Chem. Phys., 1993, 98, 2927 CrossRef PubMed.
- N. Durán and C. F. M. Menck, Crit. Rev. Microbiol., 2001, 27, 201 CrossRef PubMed.
- C. S. Castro, L. C. A. Oliveira and M. C. Guerreiro, Catal. Lett., 2009, 133, 41 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra13435d |
| This journal is © The Royal Society of Chemistry 2014 |