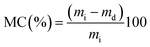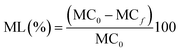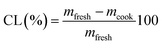Application of soy protein coatings and their effect on the quality and shelf-life stability of beef patties
Pedro Guerrero a,
Maurice G. O'Sullivanb,
Joe P. Kerryb and
Koro de la Caba*a
a,
Maurice G. O'Sullivanb,
Joe P. Kerryb and
Koro de la Caba*a
aBIOMAT Research Group, University of The Basque Country (UPV/EHU), Polytechnic School, Plaza Europa 1, 20018 Donostia-San Sebastian, Spain. E-mail: koro.delacaba@ehu.es; Tel: +34 943 017188
bFood Packaging Research Group, University College Cork (UCC), Cork, Ireland
First published on 24th December 2014
Abstract
The application of soy protein coating was found to be effective in delaying lipid oxidation and deterioration of beef patty quality during chilled storage. Protein-based edible coatings improved surface color stability of beef patties stored in modified atmosphere packaging (MAP). Additionally, texture profile analysis showed that textural parameters of soy protein-coated samples were maintained up to 14 days, in contrast to the deterioration observed for controls. Moreover, sensory analysis showed that consumers did not find any negative effect on hedonic or intensity attributes associated with beef patty samples following coating application. Therefore, soy protein coatings prepared in this study extended shelf-life, offering an alternative edible coating for the preservation of fresh food.
Introduction
The research in edible films and coatings to extend the shelf-life of food products has attracted increasing interest in recent years due to several factors such as consumer demand for high quality food,1 government demand for reducing packaging waste,2 and marketing demand for new products.3 Additionally, the food industry is constantly seeking new and improved packaging systems and materials in a further attempt to prevent deteriorative changes of food quality and consequently, extend food shelf-life.4Oxidation processes account for some of the most significant mechanisms which pertain to food spoilage. Lipid oxidation is one of the major limiting processes responsible for the reduction in food shelf-life, since it leads to off-flavor, off-odor and has been linked to oxidation reactions that cause product discoloration.5 Synthetic antioxidants have been used to prevent lipid oxidation, but the increasing demand of natural products has renewed the interest in natural polymers as raw materials for edible coatings or films due to their potential to extend shelf-life of food and reduce complexity and cost of packaging systems.6 Additionally, edible films and coatings can improve sensory attributes like color, odor, and taste. The most important properties of food for consumers are appearance, texture, and flavor. At the point of sale, the appearance is the most important factor, with a close link between this attribute and the decision to purchase.7 In the case of fresh meat, low storage temperatures combined with modified atmosphere packaging (MAP) are the preferred methods of preserving good appearance.8
MAP consists in the removal and/or replacement of the atmosphere surrounding a product before sealing in gas-barrier materials. Oxygen (O2), carbon dioxide (CO2) and nitrogen (N2) or their mixtures are regularly used in MAP.9 Typically, modified atmosphere packs containing 80% O2 and 20% CO2 are used in retail beef markets as CO2 inhibits growth of spoilage bacteria, and O2 favors the bright red color of fresh beef, which is appealing to consumers.10 In freshly cut beef, myoglobin is in the form of deoxymyoglobin, which is purplish-red in color. Following exposure to air or high O2 levels, oxygenation of myoglobin results in the formation of oxymyoglobin, conferring a bright cherry-red color to fresh beef. At early stages of storage, O2 retards formation of metmyoglobin, which is brown in color and undesirable from a consumer viewpoint.11 However, high O2 level can also promote oxidation, particularly of lipids, and membrane phospholipids are particularly susceptible to oxidation processes that cause the development of meat rancidity.
In this context, edible coatings and films can act as O2 barriers and extend food shelf-life.12 They can be formed from proteins such as gelatin13 and soy protein,14 polysaccharides such as starch,15 agar,16 and chitosan,17–19 or protein-polysaccharide blends.20,21 In the case of soy protein, it is extracted from soybeans used to obtain soy oil. During this process, soy flour is obtained as a secondary product and it can be purified to obtain soy protein concentrate (SPC) and soy protein isolate (SPI), adding value to agricultural by-products.22 Additionally, soy proteins have the ability to form films and coatings that show excellent functional properties and, among them, excellent O2 barrier.23,24 However, information on their application to foods is limited, thus the aim of this work was to investigate the effectiveness of soy protein-based edible coatings to maintain physicochemical properties of beef patties, extending the shelf-life of these fresh food products.
Materials and methods
Materials
Soy protein isolate (SPI, PROFAM 974) with 90% protein on a dry basis was supplied by ADM Protein Specialties Division (Koog aan de Zaan, Netherlands). The commercial bovine gelatin type A (bloom 200/220) was obtained from Sancho de Borja S.L. (Zaragoza, Spain). All proteins meet the quality standard for edible protein (1999/724/CE). Glycerol was food grade reagent (Panreac Química S.A., Barcelona, Spain) and used without any further purification.Beef processing, coating, and packaging
Fresh beef meat was supplied by a local meat processor. Beef was minced twice through a plate with 4 mm holes (Model P114L, Talsa, Valencia, Spain). Minced beef was formed into patties (100 g portions) using a meat former (Ministeak burger maker, O.L. Smith Co. Ltd., Italy).The edible coating solution of SPI was prepared by mixing SPI and 15 wt% gelatin (on SPI dry basis) in 100 ml distilled water. Solution was heated at 80 °C for 30 min under magnetic stirring. Then, 30 wt% glycerol (on SPI dry basis) was added and solution was maintained at 80 °C for other 30 min. Solution pH was adjusted to pH 10 with NaOH (0.1 M). Finally, solution was allowed to cool at 20 °C and the coating was applied with an air spray gun, using a nozzle cone (1.3 mm). In order to control thickness, coating time was fixed to 30 s, as reported in the method developed by Zhong et al.25
Non-coated (NC) and SPI-coated (C) beef patties were placed in polystyrene trays. Trays were flushed with 80% O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20% CO2 using a vacuum sealing unit (VS 100, Gustav Müller and Co. KG, Bad Homburg, Germany) connected to a gas mixer (Witt-Gasetechnik GmbH and Co. KG, Witten, Germany). Trays were covered and heat-sealed using a polyolefin film and subsequently stored for up to 14 days at 4 °C. Analyses of beef patties were carried out on days 0, 1, 4, 7, 10 and 14.
20% CO2 using a vacuum sealing unit (VS 100, Gustav Müller and Co. KG, Bad Homburg, Germany) connected to a gas mixer (Witt-Gasetechnik GmbH and Co. KG, Witten, Germany). Trays were covered and heat-sealed using a polyolefin film and subsequently stored for up to 14 days at 4 °C. Analyses of beef patties were carried out on days 0, 1, 4, 7, 10 and 14.
Determination of the degree of lipid oxidation
Lipid oxidation was measured using the 2-thiobarbituric acid assay as described by Siu and Draper.26 Minced beef samples (5 g) were homogenised for 2 min in 25 ml distilled water using an Ultra Turrax T25 homogeniser (Janke and Kunkel, IKA-Labortechnik, GmbH and Co., Staufen, Germany). Trichloroacetic acid (10%) (TCA) was added (25 ml) and the mixture shaken vigorously and filtered through Whatman No. 1 filter paper. In screw cap test tubes, 4 ml of clear filtrate was added to 1 ml of 0.06 M 2-thiobarbituric acid (TBA). The tubes were placed in a water bath and held at 80 °C for 90 min. The absorbance of the filtrate was measured on a UV-Vis spectrophotometer (Cary 300 Bio, Varian Instruments, CA, USA) at 532 nm against a blank containing all reagents (2 ml distilled water, 2 ml 10% TCA and 1 ml of 0.06 M TBA reagent). The malondialdehyde content of the samples was calculated using an extinction coefficient of 1.56 × 105 M−1 cm−1. Results were expressed as 2-thiobarbituric acid-reactive substances (TBARS) in mg malondialdehyde (MDA)/kg beef.Color measurement
The surface color of fresh minced beef patties was measured using a Konica Minolta CR-300 Chroma-Meter (Minolta Camera Co., Osaka, Japan). The Chroma-Meter consisted of a measuring head (CR-300), with an 8 mm diameter measuring area, a 2° standard observer, and a data processor (DP-301). The Chroma-Meter was calibrated on the CIELab color space system using a white tile (L* = 97.79, a* = −0.11, b* = 2.69). The L* value represents lightness and a* and b* values represent redness and yellowness, respectively.pH measurement
The pH values for minced beef meat were determined by direct insertion of the probe into the meat using a digital pH meter (Mettler-Toledo GmbH, Schwerzenbach, Switzerland) with a penetration glass electrode.Determination of moisture loss (ML)
The ML was determined as described by Shon and Haque.27 Samples (5 g) were weighed (mi) and then oven dried at 102 °C for 18 h, then cooled in a desiccator to room temperature and reweighed (md). The moisture content (MC) was calculated as:and ML values were calculated as follows:
where MC0 is the initial moisture content at day 0, and MCf are MC values at days 1, 4, 7, 10 and 14.
Determination of cook loss (CL)
Beef patties were cooked at 180 °C in a fan-assisted oven (Model 10 GN1/1, Zanussi Professional, Conegliano, Italy) to an internal temperature of 71 °C, measured using an internal temperature probe (Testo 110, Lenzkirch, Germany), and subsequently held at 180 °C for further 10 min. Following cooking, patties were cooled for 1 h before testing. Sample weights were recorded before (mfresh) and after cooking (mcook) and the differences in weights recorded. Before weighing, samples were wiped gently by hand with a paper towel to remove visible exudates. Calculation for cook loss was as follows:Texture profile analysis (TPA)
TPA was performed at room temperature with a Texture Analyser 16 TA-XT2i (Stable Micro Systems, Surrey, UK). Texture measurements were carried out on cooked beef patties. Patties were cut into 20 mm-diameter pieces and subjected to a two-cycle compression test using the 25 kg load cell. The samples were compressed to 40% of their original height with a 35 mm diameter cylindrical probe (SMSP/35 compression plate) and a cross-head speed of 1.5 mm s−1. Force–time data from each test were used to calculate mean values for the TPA parameters.Microbiological analysis
The total viable counts (TVC) of fresh beef patties (10 g) were determined28 using plate count agar (tryptone glucose yeast agar) (Oxoid Ltd. CM0325, Basingstoke, Hampshire, United Kingdom). Beef samples (10 g) were transferred into stomacher bags, diluted with 90 ml of maximum recovery diluent (MRD) and stomached for 2 min (Steward Stomacher 400 Lab Blender, London, UK) resulting in a 10−1 dilution used for analysis. Ten fold serial dilutions were prepared and 0.1 ml aliquots from each dilution were plated onto standard plate count agar (PCA). Plates were incubated at 4 °C for 7 days (psychrotrophic bacteria) and 30 °C for 48 h (mesophilic bacteria). Results were expressed as log10CFU (colony forming units)/g beef.Sensory evaluation of cooked beef patties
Following cooking, patties were cooled and each patty was cut into 8 portions. Prior to serving to panellists, beef samples were re-heated in an 800 W microwave (Model R216, Sharp Electronics Ltd., UK) for 10 s to release the meat odor and flavor. Samples of freshly cooked beef patties were evaluated by 25 assessors, according to the American Meat Science Association Guidelines.29 Samples were labelled with 3-digit random numbers and served in random order to panellists in individual booths. Panellists were instructed to cleanse their palates with water between samples. Sensory analysis descriptors were appearance, liking of texture and flavor, juiciness, tenderness, off-flavor, and overall acceptability. Assessors were asked to indicate their degree of liking on a 10 cm continuous line scale ranging from 0 (extremely dislike) to 10 (extremely like).Statistical analysis
Three individual experimental trials were carried out and all analyses were performed in duplicate to investigate the effect of coating treatment and storage time on parameters such as pH, surface color, lipid oxidation, texture and microbial growth. The significance of differences among samples on each day of storage was determined by analysis of variance (ANOVA). The analysis was carried out using a SPSS computer program (SPSS Statistic 20.0). Tukey's test was used for multiple comparisons between treatment means. Differences were statistically significant at the P < 0.001 level. Data is presented as mean values ± the standard error of the mean.Results and discussion
Lipid oxidation
Lipid oxidation is one of the main factors limiting the quality and acceptability of lipid-containing foods as it affects the sensory quality due to off-flavor and off-odor development.30 To estimate the extent of lipid oxidation in food products, the 2-thiobarbituric acid (TBA) test was used.31 In this study, thiobarbituric acid-reactive substances (TBARS) in non-coated and SPI-coated raw beef patties were determined over the 14 day storage period at 4 °C in MAP conditions. TBARS values are shown in Fig. 1. | ||
| Fig. 1 Effect of SPI coating on TBARS values during storage. (a–f) Columns with the same letter are not significantly (P > 0.001) different through the Tukey's multiple range test. | ||
Lipid oxidation increased over the 14 day storage time; however, there was no significant difference (P > 0.001) observed until day 10 of storage for SPI-coated patties, while TBARS values significantly increased (P < 0.001) since the first day of storage for non coated patties, reaching values next to 2 mg MDA/kg beef at the end of the storage period. In the case of SPI-coated patties, TBARS values remained under 0.5 mg MDA/kg beef up to day 14 of storage, being 60% lower than the values obtained for non-coated samples at the end of the storage period.
These results suggest that the SPI-edible coating delayed lipid oxidation by protecting meat from O2 during storage and are in accordance with those reported by Wu and Brewer,32 which showed protective effects of soy protein isolate against induced lipid oxidation in beef model systems. This antioxidant effect can be explained by two factors. On one hand, the amino acids such as cysteine, tyrosine, tryptophan and histidine in SPI are known to be free radical scavengers.33 On the other hand, the antioxidant effect of SPI-coatings is strongly linked to their low O2 permeability.34 This antioxidant effect of SPI has been recently reported by Danowska-Oziewicz35 for low-fat pork patties, regardless of the storage conditions employed, freezing, MAP or vacuum storage.
Surface color stability
Color change is also an important factor influencing the acceptability of food products, thus color tends to be used as an indicator of quality and freshness of food, and it is regarded as the first limiting factor in the shelf-life of beef. As can be seen in Fig. 2, there was no difference in the appearance of fresh beef patties after coating, which is of great importance from the consumers point of view when purchasing meat.Surface color measurements of raw beef patties were determined using CIELab system. As can be seen in Table 1, there was no significant difference (P > 0.001) in L* values, which represent the surface lightness, with treatment or storage period. However, b* values slightly decreased with time, although there is no significant difference (P > 0.001) between coated and non-coated samples. Finally, a* values, which indicate the surface redness, significantly decreased (P < 0.001) since the first day of storage for non-coated samples, while these values did not significantly changed (P > 0.001) until the day 7 of storage for SPI-coated patties. This delay in color deterioration is in accordance with the delay in lipid oxidation measured by TBARS values.
| Treatment | Parameter | Storage time at 4 °C (days) | |||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 4 | 7 | 10 | 14 | ||
| a (a–f) Two means followed by the same letter in the same parameter are not significantly (P > 0.001) different through the Tukey's multiple range test. | |||||||
| Non-coated | L* | 46.87 ± 2.89a | 47.37 ± 1.83a | 47.38 ± 0.73a | 49.14 ± 2.12a | 48.30 ± 2.85a | 50.99 ± 2.54a |
| SPI-coated | 45.56 ± 1.63a | 47.19 ± 1.53a | 45.76 ± 2.55a | 48.43 ± 1.29a | 47.15 ± 2.18a | 50.21 ± 2.27a | |
| Non-coated | a* | 25.06 ± 0.88b | 24.65 ± 0.66b | 20.89 ± 0.38c | 19.42 ± 0.32d | 16.86 ± 0.55e | 10.60 ± 0.57f |
| SPI-coated | 23.50 ± 0.88a | 24.11 ± 0.52a | 23.01 ± 0.36a | 19.88 ± 0.49c | 18.42 ± 0.48d | 11.72 ± 0.60f | |
| Non-coated | b* | 15.86 ± 1.19a | 14.67 ± 0.70ab | 13.68 ± 0.26bc | 13.07 ± 0.30bc | 12.39 ± 0.60c | 12.04 ± 0.61c |
| SPI-coated | 14.32 ± 0.74ab | 14.20 ± 0.69ab | 13.77 ± 0.36b | 12.24 ± 0.43c | 12.41 ± 0.58c | 12.07 ± 0.62c | |
The color of meat depends on many factors such as concentration of pigments, particularly myoglobin, and the chemical state of these pigments. Myoglobin combines with oxygen to form bright red oxymyoglobin, which is thought to indicate. The loss of the bright red color is caused by the oxidation of the oxymyoglobin to the undesirable brown metmyoglobin, but discoloration in retail meats during display conditions may occur as a combined function of muscle pigment oxidation (oxymyoglobin to metmyoglobin) and lipid oxidation in membrane phospholipids. Under MAP conditions, the ratio of oxymyoglobin to metmyoglobin depends on the amount of oxygen present. High concentrations of oxygen, such as the 80% O2 used in this study, favor the presence of oxymyoglobin as the dominant pigment form, and autooxidation is minimised in the first days of storage.36
pH, moisture content (MC), moisture loss (ML) and cook loss (CL)
Lactic acid bacteria have been found to be the dominant biota in fresh meat stored under MAP conditions, resulting in a decrease of meat pH during storage.37 Initial pH values and their evolution during chilled storage of non-coated and SPI-coated patties are shown in Table 2. There was no significant difference (P > 0.001) between the initial pH values measured for treated and untreated samples. These initial values were common pHs for meat, 5.81 ± 0.07 and 5.89 ± 0.03 for non-coated and SPI-coated beef patties, respectively. However, pH values significantly decreased (P < 0.001) from day 1 of storage for non-coated samples, while pH did not significantly change (P > 0.001) for SPI-coated samples until day 4 of storage. The decrease of pH values observed during storage time can be due to CO2, which is water and lipid soluble and can dissolve into meat until saturation or equilibration is reached under MAP conditions.38| Treatment | Parameter | Storage time at 4 °C (days) | |||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 4 | 7 | 10 | 14 | ||
| a (a–c) Two means followed by the same letter in the same parameter are not significantly (P > 0.001) different through the Tukey's multiple range test. | |||||||
| Non-coated | pH | 5.81 ± 0.07a | 5.68 ± 0.12b | 5.61 ± 0.03b | 5.58 ± 0.05bc | 5.53 ± 0.12bc | 5.43 ± 0.08c |
| SPI-coated | 5.89 ± 0.03a | 5.75 ± 0.02a | 5.68 ± 0.04b | 5.67 ± 0.05b | 5.66 ± 0.02b | 5.44 ± 0.04c | |
| Non-coated | MC (%) | 70.99 ± 0.40a | 70.78 ± 0.25a | 69.30 ± 0.54ab | 68.95 ± 1.39b | 68.32 ± 0.63b | 67.55 ± 0.39b |
| SPI-coated | 70.54 ± 0.32a | 70.13 ± 0.14a | 70.05 ± 0.54a | 69.30 ± 1.39ab | 68.43 ± 0.64b | 68.12 ± 0.37b | |
| Non-coated | ML (%) | 0.29 ± 0.13a | 2.37 ± 0.44ab | 2.87 ± 0.46ab | 3.76 ± 0.32b | 4.84 ± 0.21b | |
| SPI-coated | 0.59 ± 0.08a | 0.69 ± 0.13a | 1.75 ± 0.05a | 2.99 ± 0.23ab | 3.43 ± 0.27b | ||
| Non-coated | CL (%) | 31.85 ± 0.62a | 31.24 ± 1.07a | 34.51 ± 1.41a | 34.88 ± 1.14a | 34.03 ± 3.30a | 35.37 ± 2.53a |
| SPI-coated | 30.50 ± 1.29a | 32.09 ± 1.34a | 33.98 ± 0.98a | 32.87 ± 0.61a | 34.84 ± 3.30a | 32.86 ± 2.02a | |
Moisture change is another critical factor to take into account when analysing physicochemical changes in fresh foods since it affects the quality and shelf life of food. In this work, there was no significant difference (P > 0.001) on MC for SPI-coated samples up to day 7 of storage, while the decrease in MC was significant (P < 0.001) at day 4 of storage for untreated samples. These differences were noticeable in ML values, which decreased for both coated and non-coated patties, but the decrease was significant (P < 0.001) on day 4 of storage for untreated samples, while remained with no significant change (P > 0.001) until day 10 of storage for SPI-coated patties.
Finally, weight loss after cooking was measured to determine CL since this value can have negative effect on meat and affects acceptability. It is worth noting that neither storage time nor treatment had a significant effect (P > 0.001) on CL values.
Texture profile analysis (TPA)
Human perception of meat palatability is derived from the interaction of sensory and mechanical processes during chewing. In this study, the mechanical process of mastication was simulated using texture profile analysis during two cycles of deformation. The resulting data are shown as force–time in Fig. 3.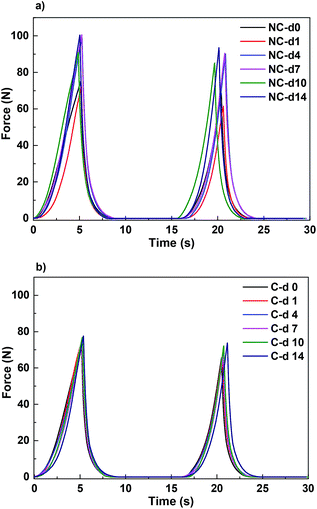 | ||
| Fig. 3 Force–time plots to determine texture parameters for (a) non coated (NC) and (b) SPI-coated (C) samples during the 14 day period of storage. | ||
According to Bourne,39 the maximum force required for the first compression measures hardness; the ratio of the time duration of force input during the second compression to that during the first compression determines springiness; the value of cohesiveness was obtained from the ratio of the positive force area during the second compression to that during the first compression; and chewiness is hardness multiplied by cohesiveness multiplied by springiness. Taking the above into consideration, it can be concluded that the edible coating prepared in this study prevented textural loss during storage since there was no change in the force–time curves, as can be observed in Fig. 2. Conversely, non-coated samples showed an increase in both intensity and peak area, thereby indicating a deterioration in patty textural parameters during chilled storage.
Textural parameters were determined from the force-deformation curves obtained during the two deformation cycles of cooked patties as described above, and values are shown in Table 3.
| Treatment | Parameter | Storage time at 4 °C (days) | |||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 4 | 7 | 10 | 14 | ||
| a (a and b) Two means followed by the same letter in the same parameter are not significantly (P > 0.001) different through the Tukey's multiple range test. | |||||||
| Non-coated | Hardness (N) | 75.04 ± 2.51a | 74.32 ± 3.05a | 98.71 ± 4.08b | 99.69 ± 5.14b | 98.03 ± 3.45b | 98.16 ± 1.42b |
| SPI-coated | 72.45 ± 1.16a | 72.51 ± 2.19a | 72.82 ± 2.52a | 73.05 ± 2.72a | 76.35 ± 3.22a | 78.03 ± 2.08a | |
| Non-coated | Springiness (mm) | 0.86 ± 0.03a | 0.86 ± 0.03a | 0.86 ± 0.09a | 0.87 ± 0.08a | 0.87 ± 0.08a | 0.87 ± 0.07a |
| SPI-coated | 0.85 ± 0.06a | 0.85 ± 0.05a | 0.85 ± 0.04a | 0.85 ± 0.03a | 0.85 ± 0.02a | 0.85 ± 0.09a | |
| Non-coated | Cohesiveness | 0.70 ± 0.09a | 0.70 ± 0.09a | 0.71 ± 0.06a | 0.71 ± 0.03a | 0.71 ± 0.03a | 0.72 ± 0.04a |
| SPI-coated | 0.70 ± 0.08a | 0.70 ± 0.08a | 0.70 ± 0.06a | 0.70 ± 0.06a | 0.70 ± 0.04a | 0.71 ± 0.03a | |
| Non-coated | Gumminess (N) | 51.34 ± 3.47a | 51.45 ± 2.88a | 65.61 ± 3.91ab | 66.91 ± 4.47b | 68.01 ± 4.63b | 69.68 ± 4.74b |
| SPI-coated | 50.81 ± 2.27a | 50.85 ± 2.58a | 50.91 ± 1.31a | 51.84 ± 1.34a | 58.51 ± 4.38ab | 60.51 ± 2.83ab | |
| Non-coated | Chewiness (N mm) | 45.89 ± 2.55a | 45.95 ± 3.78a | 55.95 ± 3.77a | 56.62 ± 3.96a | 58.29 ± 3.94a | 60.62 ± 3.54a |
| SPI-coated | 44.55 ± 2.92a | 44.86 ± 2.81a | 45.02 ± 4.29a | 46.35 ± 3.31a | 49.68 ± 3.04a | 51.32 ± 3.85a | |
It is worth noting that there is no significance difference (P > 0.001) in the initial textural parameters measured for both coated and non-coated samples, indicating that SPI-coating did not adversely affect the tenderness of beef patties. As can be deduced from the force–time curves, there is no significant difference (P > 0.001) in hardness, springiness, cohesiveness or chewiness for SPI-coated samples. However, hardness and gumminess significantly increased (P < 0.001) from day 4 of storage for non-coated samples. As previously observed by other authors,40 lipid oxidation not only causes the development of food rancidity, but also affects texture. The results obtained from TPA confirm the positive effect of SPI-coating on the quality attributes of beef patties.
Sensory analysis
In addition to objective textural parameters, subjective sensory characteristics of beef patties were determined by sensory panels. Sensory tests were split into two parts in order to rate the quality of beef patties. Firstly, hedonic characteristics such as appearance of raw and cooked patties were used to assess liking of texture, liking of favor, and overall acceptability; secondly, intensity assessment was used to evaluate patty attributes such as juiciness, tenderness and off-flavors. The evolution of these sensory attributes is shown in Fig. 4. | ||
| Fig. 4 Effect of the treatment on sensory attributes scores for (a) non coated (NC) and (b) SPI-coated (C) samples on day 1 and 7 of storage. | ||
Sensory scores for off-flavor were very low for all samples. It is also worth noting that there was no significant change between coated and non-coated samples, indicating that the application of the SPI-coating on the surface of beef patties to extend shelf-life had no adverse effect on consumer evaluation. In order to study the textural relationship between instrumental and sensory assessments of beef patties, Pearson correlation analysis was carried out on the relevant data generated. Pearson correlation coefficients between sensory variables are shown in Table 4.
| App. (raw) | App. (cook) | Texture | Flavor | Acceptability | Juiciness | Tenderness | |
|---|---|---|---|---|---|---|---|
| App. (Cook) | 0.6209 | ||||||
| Texture | 0.4632 | 0.4852 | |||||
| Flavor | 0.4216 | 0.5926 | 0.4851 | ||||
| Acceptability | 0.5553 | 0.4533 | 0.6743 | 0.7674 | |||
| Juiciness | 0.3851 | 0.3220 | 0.6111 | 0.2964 | 0.4507 | ||
| Tenderness | 0.3237 | 0.3479 | 0.5176 | 0.4277 | 0.4070 | 0.3406 | |
| Off-flavor | −0.2398 | −0.2252 | −0.1433 | −0.1794 | −0.1626 | −0.1687 | 0.0844 |
In general, all variables were closely related to each other. Overall acceptability was positively correlated with appearance, texture, and flavor. Texture was also positively correlated with juiciness and tenderness as well as the appearance of cooked patties with flavor. Finally, off-flavor was negatively related to all other test variables.
Total viable counts (TVC)
Microbial growth is generally responsible for the spoilage in refrigerated foods and alongside oxidation processes constitutes another of the primary mechanisms for food product deterioration. Total viable counts (TVC) for coated and non-coated samples during refrigerated storage are shown in Fig. 5.A significant increase (P < 0.001) in TVC was observed with storage time for both coated and non-coated samples, and with the exception of initial values, psychotropic counts were higher than mesophilic counts, which may be attributed to refrigeration temperatures that encourage the growth of psychotropic bacteria and retard the growth of mesophilic bacteria. In the present study, TVC values were all below log10CFU g−1 7.0, thereby indicating that it was acceptable from a microbiological perspective.41
Although TVC values were slightly lower for SPI-coated samples compared to non-coated equivalents, no significant difference (P > 0.001) was observed between treated and untreated samples. Therefore, SPI coating did not inhibit the growth of spoilage microorganisms during storage, indicating that SPI was not an effective antimicrobial coating. These results are in accordance with previous research that found that SPI-based edible films with oregano or thyme essential oils did not have significant effects on TVC when applied on beef patties.42
Conclusions
SPI-based edible coating was effective in delaying lipid oxidation and color deterioration, thereby extending the shelf life of fresh beef patties. Moreover, the coating maintained textural parameters during storage and did not affect sensory attributes negatively. These beneficial results can be attributed to the excellent O2 barrier properties of soy protein and to the ability of radical scavenging by some amino acids present in soy protein. Therefore, results confirm the potential of soy protein coating as antioxidant coating to improve the quality of fresh foods.Acknowledgements
The authors thank the University of the Basque Country UPV/EHU (research group GIU12/06), the Basque Government (projects S-PE12UN002 and S-PE13UN057), and the Provincial Council of Gipuzkoa (OF144/2014 (B)) for their financial support. Pedro Guerrero and Koro de la Caba thank Mr Eddie Beatty and Mr James Mcnamara for their technical and human support.References
- R. Andrade, O. Skurtys and F. Osorio, Food Res. Int., 2013, 54, 397–405 CrossRef CAS PubMed.
- I. Leceta, A. Etxabide, S. Cabezudo, K. de la Caba and P. Guerrero, J. Cleaner Prod., 2014, 64, 218–227 CrossRef CAS PubMed.
- L. Angiolillo, A. Conte, M. Faccia, A. V. Zambrini and M. A. Del Nobile, Innovative Food Sci. Emerging Technol., 2014, 22, 180–187 CrossRef CAS PubMed.
- T. Karbowiak, F. Debeaufort, A. Voilley and G. Trystram, Innovative Food Sci. Emerging Technol., 2009, 10, 116–127 CrossRef CAS PubMed.
- F. Tiang, E. A. Decker and J. M. Goddard, Food Funct., 2013, 4, 669–680 Search PubMed.
- T. Janjarasskul and J. M. Krochta, Annu. Rev. Food Sci. Technol., 2010, 1, 415–448 CrossRef CAS PubMed.
- C. E. Carpenter, D. P. Cornforth and D. Whittier, Meat Sci., 2001, 57, 359–363 CrossRef CAS.
- P. I. Zakrys-Waliwander, M. G. O'Sullivan, E. E. O'Neill and J. P. Kerry, Food Chem., 2012, 131, 527–532 CrossRef CAS PubMed.
- P. Singh, A. A. Wani, S. Saengerlaub and H. C. Langowski, Crit. Rev. Food Sci. Nutr., 2011, 51, 146–177 CrossRef PubMed.
- Y. H. Kim, E. Huff-Lonergan, J. G. Sebranek and S. M. Lonergan, Meat Sci., 2010, 85, 759–767 CrossRef CAS PubMed.
- S. Jonberg, S. H. Skov, M. A. Torngren, L. H. Skibsted and M. N. Lund, Food Chem., 2011, 128, 276–283 CrossRef PubMed.
- Y. Xing, Q. Xu, Z. Che, X. Li and W. Li, Food Funct., 2011, 2, 466–474 CAS.
- P. W. Gordon, A. D. M. Brooker, Y. M. J. Chew, D. I. Wilson and D. W. York, Food Bioprod. Process., 2010, 88, 357–364 CrossRef CAS PubMed.
- P. Guerrero, Z. A. Nur Hanani, J. P. Kerry and K. de la Caba, J. Food Eng., 2011, 107, 41–49 CrossRef CAS PubMed.
- M. Chiumarelli and M. D. Hubinger, Food Hydrocolloids, 2012, 28, 59–67 CrossRef CAS PubMed.
- P. Guerrero, T. Garrido, I. Leceta, M. Peñalba and K. de la Caba, Carbohydr. Polym., 2014, 99, 491–498 CrossRef CAS PubMed.
- I. Leceta, P. Guerrero, I. Ibarburu, M. T. Dueñas and K. de la Caba, J. Food Eng., 2013, 116, 889–899 CrossRef CAS PubMed.
- I. Leceta, S. Molinaro, P. Guerrero, J. P. Kerry and K. de la Caba, Postharvest Biol. Technol., 2015, 100, 142–150 CrossRef CAS PubMed.
- Y. Xing, Q. Xu, Z. Che, X. Li and W. Li, Food Funct., 2011, 2, 466–474 CAS.
- D. Jia, Y. Fang and K. Yao, Food Bioprod. Process., 2009, 87, 7–10 CrossRef CAS PubMed.
- L. Z. Wang, L. Liu, J. Holmes, J. F. Kerry and J. P. Kerry, Int. J. Food Sci. Technol., 2007, 42, 1128–1138 CrossRef CAS PubMed.
- T. Garrido, A. Etxabide, I. Leceta, S. Cabezudo, K. de la Caba and P. Guerrero, J. Cleaner Prod., 2014, 64, 228–233 CrossRef CAS PubMed.
- S. Kokoszka, F. Debeaufort, A. Hambleton, A. Lenart and A. Voilley, Innovative Food Sci. Emerging Technol., 2010, 11, 503–510 CrossRef CAS PubMed.
- S. Galus, H. Mathieu, A. Lenart and F. Debeaufort, Innovative Food Sci. Emerging Technol., 2012, 16, 148–154 CrossRef CAS PubMed.
- Y. Zhong, G. Cavender and Y. Zhao, LWT--Food Sci. Technol., 2014, 56, 1–8 CrossRef CAS PubMed.
- G. M. Siu and H. H. Draper, J. Food Sci., 1978, 43, 1147–1149 CrossRef CAS PubMed.
- J. Shon and Z. U. Haque, J. Food Qual., 2007, 30, 581–593 CrossRef CAS PubMed.
- ISO 4833:2003, National Standards Authority of Ireland, 2003.
- AMSA 2005, American Meat Science Association in Cooperation with National Live Stock and Meat Board, 2005.
- J. E. Hayes, V. Stepanyan, P. Allen, M. N. O'Grady and J. P. Kerry, LWT--Food Sci. Technol., 2011, 44, 164–172 CrossRef CAS PubMed.
- F. Shahidi, in Flavor of Meat and Meat Products, ed. F. Shahidi, Blackie Academic & Professional, 1994, pp. 247–266 Search PubMed.
- S. Y. Wu and M. S. Brewer, J. Food Sci., 1994, 59, 702–706 CrossRef CAS PubMed.
- H. K. Kang, S. J. Kim, Y. S. You, M. Lacroix and J. Han, LWT--Food Sci. Technol., 2013, 51, 393–396 CrossRef CAS PubMed.
- J. Bonilla, L. Atares, M. Vargas and A. Chiralt, J. Food Eng., 2012, 110, 208–213 CrossRef CAS PubMed.
- M. Danowska-Oziewicz, J. Food Process. Preserv., 2014, 38, 641–654 CrossRef CAS PubMed.
- J. P. Kerry, M. G. O'Sullivan, D. J. Buckley, P. B. Lynch and P. A. Morrissey, Meat Sci., 2000, 56, 61–66 CrossRef CAS.
- A. I. Doulgeraki, S. Paramithiotis, D. M. Kagkli and G. J. E. Nychas, Food Microbiol., 2010, 27, 1028–1034 CrossRef PubMed.
- J. M. Rodríguez-Calleja, M. C. Cruz-Romero, M. G. O'Sullivan, M. L. García-López and J. P. Kerry, Food Control, 2012, 25, 516–524 CrossRef PubMed.
- M. C. Bourne, Food Tech., 1978, 33, 62–66 Search PubMed.
- P. Gatellier, C. Hamelin, Y. Durand and M. Renerre, Meat Sci., 2001, 59, 133–140 CrossRef CAS.
- S. P. Verma and J. Sahoo, Meat Sci., 2000, 56, 403–413 CrossRef CAS.
- Z. K. Emiroğlu, G. P. Yemiş, B. K. Coşkun and K. Candoğan, Meat Sci., 2010, 86, 283–288 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2015 |