In vivo pharmacokinetic features and biodistribution of star and rod shaped gold nanoparticles by multispectral optoacoustic tomography
Jing Wang†
a,
Yadian Xie†ab,
Liming Wanga,
Jinglong Tanga,
Jiayang Li*a,
Duygu Kocaefeb,
Yasar Kocaefeb,
Zhiwen Zhangc,
Yaping Lic and
Chunying Chen*a
aCAS Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety, National Center for Nanoscience and Technology, Beijing 100190, China. E-mail: chenchy@nanoctr.cn; lijiayang@nanoctr.cn; Fax: +86-10-6265-6765; Tel: +86-10-8254-5560
bDepartment of Applied Sciences, University of Quebec at Chicoutimi (UQAC), Canada
cShanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai, 201203, China
First published on 22nd December 2014
Abstract
Metallic nanomaterials, especially gold nanoparticles, which have low toxicity and high photothermal conversion efficiency, exhibit promising theranostic applications in biomedical fields. However, detailed preclinical studies of these nanomaterials, such as pharmacokinetics, metabolism, long-term stability and toxicity potential should still be performed. Photoacoustic imaging as a novel non-invasive imaging technique provides convenience for studying real-time semiquantitative pharmacokinetic and biodistribution profiles of drugs or probes to determine whether they can reach the target areas and their metabolic pathways. In this article, we prepared chitosan-capped star shaped gold nanoparticles (AuNSs) and used multispectral optoacoustic tomography (MSOT) to visualize AuNSs in the blood vessels, liver, spleen and kidneys in real time. We also compared these results with chitosan-capped rod shaped gold nanoparticles (AuNRs). The results show that both AuNSs and AuNRs experience rapid blood clearance and have fast and long-term accumulation in the liver and spleen (reaching the peak value within 10 min). The pharmacokinetic data of the two gold nanoparticles fitted to the two-compartment model. The important pharmacokinetic parameters were calculated. The results from MSOT were qualitatively validated by inductively coupled plasma mass spectrometery (ICP-MS). Our results demonstrate that MSOT can serve as an effective tool to monitor gold nanoparticles' pharmacokinetics and biodistribution in specific organs.
Introduction
With the rapid development of nanotechnology, the application of nanomaterials in medicine shows potent improvements in early diagnosis and therapies of many diseases of global concern, such as cardiovascular disease, Alzheimer disease, and cancer.1–4 Due to their unique physicochemical properties, metallic nanomaterials responsive to near-infrared (NIR, 650–900 nm) light have generated significant interests for biomedical applications in recent years.5,6 Since NIR light is minimally absorbed by skin and tissue, it has deeper tissue penetration and less tissue invasion.7 Thus these metallic nanomaterials, especially gold nanoparticles, which have high-absorption cross section for conversion of NIR light to heat, exhibit diverse and promising theranostic applications in multimodal imaging, local hyperthermia, and drug delivery.8–12 Our recent studies exhibit an innovative and effective strategy for targeted cancer therapy in nanomedicine by developing a NIR light-triggered nanoplatform of thermoresponsive polymer encapsulated mesoporous silica coated-gold nanorod for site-specific hyperthermia and light-controllable drug delivery.13 In spite of having a vast application prospect, reports on clinical applications of these metallic nanoparticles are still far from satisfactory. Widely as gold nanoparticles have been recognized for their low toxicity, good biocompatibility and chemical inertness,14 detailed preclinical studies, such as pharmacodynamics, pharmacokinetics (absorption, distribution, metabolism and excretion), long-term stability, and toxicity evaluation, should be performed which are important and essential for these new potential nanomedicine candidates before their clinical trials.15The traditional methods of studying in vivo pharmacokinetic and biodistribution need to collect the blood at different time points or to remove some part of the viscera for in vitro physicochemical analysis, which are time-consuming and high-cost. Multiple imaging techniques, such as optical imaging, positron emission tomography (PET), X-ray computed tomography (CT), magnetic resonance imaging (MRI) and photoacoustic imaging, have provided non-invasive pharmacokinetic and biodistribution analysis of drugs or probes.16 Conventional optical imaging suffers from low spatial resolution and shallow penetration depth (∼1 mm) which limits its application.17,18 PET requires radioactive-labeling materials that may have potential hazards to patients.19 CT and MRI have higher spatial resolution (CT: ∼10–50 μm; MRI: 100 μm) than optical imaging and PET (∼1 mm), but it cannot achieve dynamic pharmacokinetic and biodistribution analysis in real time.20,21 Furthermore, the cost of MRI is very expensive. Photoacoustic imaging as a novel biomedical imaging technique has recently attracted significant attention and research interests.22–25 It takes advantage of both optical and acoustic imaging with deeper tissue penetration (up to 40 mm), higher spatial resolution (150 μm for whole body; 20–50 μm for local imaging), and higher contrast. In photoacoustic imaging technology, multispectral optoacoustic tomography (MSOT) is a convenient and efficient tool. By using multiple excitation wavelengths and multispectral unmixing, images of both tissue-intrinsic (e.g. hemoglobin, melanin) and extrinsic (e.g. fluorescent dyes, probes and gold nanoparticles) absorbers can be acquired at the same time, supplying rich information for disease diagnosis and analysis. Moreover, as a complementary tool with other imaging techniques, MSOT provides a real-time monitoring method to evaluate photothermal-responsive gold nanomaterials' pharmacokinetics and biodistribution. Especially for those gold nanoparticles with short blood circulation time and fast distribution in vivo, MSOT will present more detailed information for their early changes of pharmacokinetic features and biodistribution, which helps guide their clinical applications.
The biological effects of gold nanoparticles with various surface modifications, sizes and shapes have drawn an increased interest both in vitro and in vivo.26–33 For example, our group found that poly(diallyldimethylammonium chloride) (PDDAC) coated gold nanoparticles have exhibited more than 10 folds cellular uptake than cetyltrimethylammonium bromide (CTAB) coated gold nanoparticles with almost no cytotoxicity.26 Jong et al. indicated that 10 nm gold nanoparticles showing the most widespread organ distribution than other larger particles which are only detected in the blood, liver and spleen.30 Arnida et al. reported that poly(ethylene glycol) (PEG) functionalized gold nanorods are taken up to a lesser extent by the liver and had longer circulation time in the blood compared with their spherical counterparts.32 Huang et al. found that short-rod mesoporous silica nanoparticles (short-rod MSNs, aspect ratios: 1.5) are easily trapped in the liver while long-rod mesoporous silica nanoparticles (long-rod MSNs, aspect ratios: 5) are more distributed in the spleen at 2 h post injection, and short-rod MSNs have a more rapid clearance rate than long-rod MSNs by urine and feces.33 Recently, star shaped gold nanoparticles (AuNSs) have been rapidly developed for biomedical applications as a novel photothermal platform due to the fact that elongated or sharp nanoparticles appear to be more efficient heaters than other nanostructures.34,35 AuNSs can also be used as carriers for drugs or photosensitizers with high loading efficiency by taking advantage of their high surface-to-volume ratio.36,37 However, data on pharmacodynamics and pharmacokinetics studies of AuNSs are limited in the literature. This study aims to evaluate the pharmacokinetic and biodistribution of AuNSs as new MSOT contrast agents, and to compare their results with rod shaped gold nanoparticles (AuNRs), which has been widely used in biomedical applications. Chitosan was chosen as the surface stabilizer of AuNSs and AuNRs which has favourable properties such as biocompatibility, non-toxicity and biodegradability and has been widely applied in drug delivery.38,39 In this article, we present a MSOT method for real-time visualization of chitosan-capped AuNSs in blood vessels, liver, spleen and kidney, respectively, compared to AuNRs with the same surface coating (Scheme 1).
 | ||
| Scheme 1 Schematic illustration of pharmacokinetic and biodistribution analysis of chitosan-capped star or rod shaped gold nanoparticles by multispectral optoacoustic tomography. | ||
Materials and methods
Chemicals and materials
Hydrogen tetrachloroaurate(III) trihydrate (HAuCl4·3H2O, 99.9%), silver nitrate (AgNO3, 99%), ascorbic acid (AA, 99%), glucose (99%), sodium borohydride (NaBH4), cetyltrimethylammonium bromide (CTAB) were purchased from Alfa Aesar, United States. Chitosan (low molecular weight, 99%) was purchased from Aldrich Chemical, United States. HNO3 (MOS), H2O2 (MOS), ethylic acid, hydrochloric acid (HCl, GR 36.0–38.0%) were purchased from Beijing Chemical Reagent Institute, China. Isoflurane was purchased from Hebei Jiuzhi Pharmaceutical Limited Company, China. All the chemicals and materials used were of analytical grade unless otherwise stated. Stock standard solutions for gold and bismuth were obtained as 1000 mg L−1 from the National Research Centre for CRMs, China. Ultrapure Milli-Q water with resistivity of 18.2 MΩ was used for all solution preparations.Apparatus
Transmission electron microscope (TEM) images were acquired on a FEI Tecnai G2 F20 U-TWIN transmission electron microscope (FEI Company, USA). Scanning electron microscope (SEM) measurements were performed on a Hitachi S-4800 field emission scanning electron microscope (Hitachi Ltd., Japan). Hydrodynamic diameter and zeta-potential measurements were taken using a Malvern zeta sizer Nano ZS instrument (Malvern Instruments, United Kingdom). Extinction spectrum was obtained using a UV-3600 spectrophotometer (Shimadzu Corporation, Japan). Photoacoustic imaging was performed on MSOT inVision 128 (iThera Medical, Germany). The amount of Au was accurately measured by an Elan DRC II inductively coupled plasma mass spectrometer (ICP-MS, Perkin-Elmer, USA).Synthesis of AuNSs and AuNRs
AuNSs were synthesized as described in the literature.40 Briefly, AuNSs were obtained by mixing 2 mL 10 mM HAuCl4 aqueous solution with 100 mL H2O and 0.2 mL 10 mM AgNO3 aqueous solution. Then 0.4 mL 100 mM AA aqueous solution was rapidly added to the mixed solution with stirring at 400 rpm for 10 s. After one minute at rest, 100 mL 1 wt% chitosan and 1 wt% ethylic acid aqueous solution was added to the above solution to stabilize AuNSs. The color of the solution change from yellow to colorless after the reducing agent was added, and finally became light blue.CTAB-capped AuNRs were synthesized by the seed-mediated growth method in our previous work.12 Chitosan-coated AuNRs were prepared by using 1 wt% chitosan and 1 wt% ethylic acid aqueous solution to resuspend CTAB-capped AuNRs' precipitation after centrifugation. Then the solution was stirred overnight.
Characterizations
The size and morphology of gold nanoparticles were observed by TEM and SEM. Hydrodynamic diameter and zeta-potential were measured in water solution at 25 °C. The NIR extinction spectra were obtained with a 1 cm wide quartz cell at 25 °C by UV spectrometer. The MSOT-derived spectra were measured by agar phantom (1.2 wt% agar concentration in aqueous solution) as a container and acquired by PCA/ICA method of multispectral unmixing.Sensitivity study
The linearity of photoacoustic signal across concentration of AuNSs and AuNRs were tested by MSOT with agar phantom. Six gold nanoparticles' aqueous solutions with different concentrations were prepared. The concentrations of the solutions were 1.56, 3.13, 6.25, 12.5, 25 and 50 μg mL−1, which the corresponding value of maximum optical density (ODmax) was 0.025, 0.05, 0.1, 0.2, 0.4 and 0.7, respectively, according to the NIR extinction spectra results. The photoacoustic signals of the corresponding solutions were measured by MSOT with excitation wavelengths from 680 nm to 900 nm in 10 nm steps. The quantitative analysis of the spectra was unmixed by linear regression.Real-time MSOT imaging for in vivo pharmacokinetic and biodistribution study
Procedures involving animals were approved by the Institutional Animal Care and Use Committee (IACUC). The male Balb/c nude mouse (6–8 weeks, 20 g body weight) was narcotized by isoflurane and placed on a holder with thin polyethylene membrane and ultrasonic coupling agent. Then its tail vein was connected to a syringe through syringe needle (30 G syringes) and a long polythene tubing (internal diameter: 0.28 mm, external diameter: 0.61 mm). After the mouse was put into the chamber of MSOT, the scan section was chosen according to the specific position of the organ or tissue and experimental parameters were set up. Then 2 mg kg−1 AuNS–chitosan or AuNR–chitosan glucose solution (10 g glucose in 20 mL water) was injected into the mouse through tail vein and then photoacoustic signal was collected immediately. Jugular vein, liver, spleen and kidneys were monitored by four mice respectively for in vivo pharmacokinetic study. The cumulative time of each in vivo study was around 45 min. The main experimental parameters were 10 excitation wavelength steps for each slice from 680 nm to 930 nm, and 25 frames per wavelength. The MSOT signals were collected by one per 30 s without delay.Image reconstruction and multispectral processing
The tomographic images from MSOT were reconstructed using a linear model-based inversion. Size and resolution presets were 25 mm ROI (region of interest) with 100μ resolution. The low and high filter cut-off frequencies were 50 kHz and 7 MHz, respectively. The injected gold nanoparticles were resolved within tissues or organs by using multispectral unmixing of linear regression. The data of pharmacokinetic and biodistribution were treated by Winnonlin 5.2 software. The temporal evolution of the signal in individual pixels fitted into classical two-compartment pharmacokinetic model, allowing the analysis of important kinetic parameters, such as absorption half-life (t1/2α), elimination half-life (t1/2β), areas under the curve (AUC), plasma clearance (Cl) and apparent volume of distribution (Vd).Blood circulation time and biodistribution study by ICP-MS
To further validate the quantitative accuracy of MSOT, 200 μL suspensions with Au content of 0.1 mg mL−1 was intravenously injected in male Balb/c mice (6–8 weeks, ∼20 g body weight). Blood samples were collected at 3 min, 10 min, 30 min, 2 h, 5 h and 24 h post administration. The mice at 3 min, 10 min, 30 min and 24 h were sacrificed and organs including heart, liver, spleen, lung and kidneys were dissected and weighted. Each group has three mice as replicates. All the samples were stored at −80 °C before ICP-MS analysis. For ICP-MS measurement, the above samples were pre-digested overnight with 5.0 mL concentrated HNO3. Then they were mixed with 3.0 mL 30% H2O2 and digested for 2 h in open vessels on a hot plate at 50–150 °C by gradually warming. When the residual volume decreased to about 1 mL, 2–3 mL aqua regia (HNO3–HCl = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) was added to continue the digestion till the solution volume decreased to about 0.5 mL. Finally, the remaining solution was cooled and diluted to 3.0 g on the balance with a mixed acid solution containing 2% HNO3 and 1% HCl. For quantitative analysis, a series of Au standard solutions (0.5, 1, 5, 10, 50 ppb) were prepared with the mixed acid solution to obtain the standard curve. Bismuth (20 ppb) in the mixed acid solution was used as an internal standard. Quantification was carried out by external five-point calibration with internal standard correction. Both the standard solutions and the samples were measured three times by ICP-MS. The amount of Au element was expressed as percentage of the injected dose (% ID).
3) was added to continue the digestion till the solution volume decreased to about 0.5 mL. Finally, the remaining solution was cooled and diluted to 3.0 g on the balance with a mixed acid solution containing 2% HNO3 and 1% HCl. For quantitative analysis, a series of Au standard solutions (0.5, 1, 5, 10, 50 ppb) were prepared with the mixed acid solution to obtain the standard curve. Bismuth (20 ppb) in the mixed acid solution was used as an internal standard. Quantification was carried out by external five-point calibration with internal standard correction. Both the standard solutions and the samples were measured three times by ICP-MS. The amount of Au element was expressed as percentage of the injected dose (% ID).
Results and discussion
Characterizations
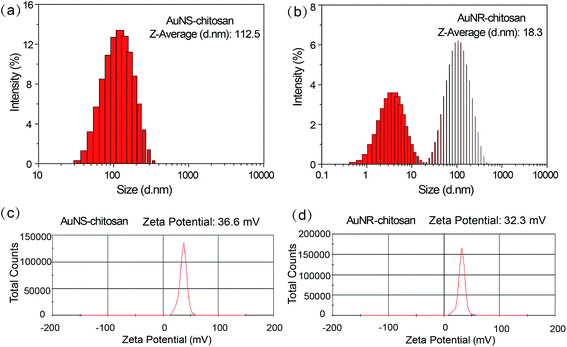
Fig. 1 shows TEM and SEM images of chitosan-capped AuNSs and AuNRs, respectively. TEM image of AuNSs show that their outer diameter was around 90 nm with 50 nm core and 20 nm tips (Fig. 1a). TEM image of AuNRs show that they had an aspect ratio of 3.2 with 18 nm width and 58 nm length (Fig. 1b). The shape of AuNSs was further confirmed by SEM image (Fig. 1c) with protruding tips on the surface of AuNSs. SEM image of AuNRs (Fig. 1d) was consistent with TEM result (Fig. 1b). Both AuNSs and AuNRs had uniform size and morphology from electron microscope results. As shown in Fig. 2a, chitosan-capped AuNSs showed an average hydrodynamic diameter of 112.5 nm with a good polydispersity index of 0.211. The size of AuNSs measured by the dynamic light scattering method was larger than that observed by TEM, because of the existence of hydration layer. Due to the rod-shaped structure, chitosan-capped AuNRs have two diffusion modes in aqueous solution, thus showed two peaks of size distribution (Fig. 2b). Zeta-potentials of AuNSs and AuNRs were measured to be +36.6 mV and +32.3 mV, respectively, due to the protonated chitosan layers (Fig. 2c and d). The surface plasmon resonance (SPR) absorbance peak of AuNSs was at ∼800 nm with a broader absorption band than AuNRs in the NIR region (Fig. 3a). AuNRs had a narrower absorption peak at 720 nm (Fig. 3a). Both absorbance curves (AuNSs and AuNRs) derived from MSOT by PCA/ICA method of multispectral unmixing were in good agreement with the UV-visible spectrometer results (Fig. 3b). The obvious NIR absorption made both AuNSs and AuNRs as a sort of very suitable NIR probes for testing by MOST. | ||
| Fig. 1 TEM images of chitosan-capped AuNSs (a) and AuNRs (b) and SEM images of chitosan-capped AuNSs (c) and AuNRs (d). | ||
Sensitivity study
Phantom measurement results showed excellent linearity (AuNSs: R2 = 0.9982; AuNRs: R2 = 0.9822) and high sensitivity (lowest limit concentration: ∼3.1 μg mL−1 Au) for both AuNSs and AuNRs probes. Six solutions of different concentrations were prepared and filled in the left hole of the phantom, while the glucose solution was as a control in the right hole (Fig. 3c and d). When the ODmax was reduced from 0.7 to 0.025, the intensity of the photoacoustic signals were decreased gradually for both AuNSs and AuNRs, showing excellent linearity of photoacoustic signal across concentration (Fig. 3e and f). Since there was no signal appearance at ODmax = 0.025 (Fig. 3c and d), the lowest detection limits for both gold nanoparticles were ∼3.1 μg mL−1 of Au (ODmax at 0.05). Besides, the intensity of photoacoustic signals at each ODmax of AuNSs was higher than that of AuNRs (Fig. 3c and d). By comparing the slope of the equation of the line, the signal intensity under the same concentration of AuNSs (equation: y = 3.63x + 0.78) was higher than AuNRs (equation: y = 3.34x + 0.67) as well (Fig. 3e and f). According to the lowest detection limit, mice were injected with 1 mg kg−1 (30 times multiplied by concentration at ODmax = 0.05) of gold nanoparticles' solution and it was found that the in vivo photoacoustic signal was very weak and unstable (data are not shown). Then dosage of 2 mg kg−1 (60 times multiplied by concentration at ODmax = 0.05) was chosen for in vivo pharmacokinetic and biodistribution study, because there was strong and stable photoacoustic signal in vivo when the mice were injected 2 mg kg−1 gold nanoparticles' solution.Real-time MSOT imaging for in vivo pharmacokinetic and biodistribution study
It is vital to understand the pharmacokinetics, drug distribution in vivo and drug accumulation and metabolism in specific organs in the new drug development process. MSOT as a non-invasive imaging technology can acquire quantitative information with real-time and dynamic imaging of drug distribution in mice with high temporal and spatial resolution. Of note, MSOT has a prominent advantage to monitor short-time and real-time accumulation and metabolism in specific organs than any other in vivo imaging technique.The pharmacokinetic analysis of gold nanoparticles was carried out by monitoring of jugular vein in the bloodstream after intravenous injection (Fig. 4). The plasma drug concentration versus time profiles of chitosan-capped AuNSs and AuNRs revealed a rapid initial concentration drop followed by more gradual decline (Fig. 4a and b). At 3 min, the percentage concentration of AuNSs remained in blood was 28% and then dropped to 8% after 40 min post administration, while the percentage concentration of AuNRs at 3 min was 20% and dropped to 3% after 40 min post administration. These pharmacokinetic characteristics are in agreement with previous reports indicating that positively charged nanoparticles possessed relatively short half-lives than neutral and zwitterionic nanoparticles.41 The pharmacokinetic data of the two gold nanoparticles fit to classical two-compartment model. The pharmacokinetic equations are C(t) = 20.11e−0.577t + 5.94e−0.018t for AuNSs and C(t) = 24.69e−0.871t + 4.12e−0.020t for AuNRs, respectively. The main pharmacokinetic parameters were listed in Table 1. It can be clearly seen that AuNSs has a longer circulation time (t1/2α ∼ 1.2 min) than AuNRs (t1/2α ∼ 48 s) in the bloodstream. Besides, AuNSs (t1/2β ∼ 38.5 min, Cl = 0.11 mL min−1) also eliminated more slowly from blood than AuNRs (t1/2β ∼ 34.7 min, Cl = 0.17 mL min−1) due to the longer elimination half-life and slower plasma clearance. Meanwhile, the value of AUC of AuNSs (AUC: 365 μg min mL−1) markedly increased by about 1.6-fold in comparison to that of AuNRs (AUC: 234 μg min mL−1). This also illustrates that the blood circulation time of AuNSs is obviously longer than AuNRs. The large values of Vd were far beyond the volume of the mouse's body fluid, which implies that the two gold nanoparticles may have a wide distribution or accumulation in the body. The smaller Vd value of AuNSs (Vd: 6.16 mL) means that more AuNSs were in the blood than AuNRs (Vd: 8.55 mL) in accord with their longer plasma half-life.
| Chitosan-capped AuNSs | Chitosan-capped AuNRs | |
|---|---|---|
| t1/2α | 1.2 min | 48 s |
| t1/2β | 38.5 min | 34.7 min |
| AUC | 365 μg mL−1 min−1 | 234 μg mL−1 min−1 |
| Cl | 0.11 mL min−1 | 0.17 mL min−1 |
| Vd | 6.16 mL | 8.55 mL |
Then we used MSOT to monitor the accumulation and metabolism of gold nanoparticles in the liver, spleen and kidney in real time. The region of interest (ROI) was chosen at the position of the liver or spleen and highlighted in yellow color circle (Fig. 5f and g). By monitoring the signal strength of ROI, the concentration–time curve of the liver after the injection of AuNSs was obtained (Fig. 5a). Both AuNSs and AuNRs have similar accumulation results in the liver. The MSOT signals showed increased accumulation in the liver as time elapsed and the concentration in the liver went up to 80% in just 5 min (Fig. 5a and b). Then the percentage concentrations of AuNSs and AuNRs reached a plateau at about 20 min and became stable in the rest of time (Fig. 5a and b). There was certain difference of accumulation between AuNSs and AuNRs in the spleen (Fig. 5c and d). The concentration of AuNSs accumulated in the spleen rapidly reached 90% within 2 min, and began to decline to about 70% in the next 15 minutes and then became stable. Meanwhile, the concentration of AuNRs in spleen rose up to 90% after 10 minutes and had a slight decline in the next 30 min. It may be attributed to the differences of shape and size or more AuNSs in the bloodstream which caused the concentration change in the spleen in the first few minutes, due to the fact that AuNSs had longer blood circulation time than AuNRs. There were no clear optoacoustic signals observed in kidneys for both AuNSs and AuNRs due to their lower concentrations (<3% of % ID, Fig. 6d) compared to liver (∼60% of % ID, Fig. 6b) and spleen (∼6% of % ID, Fig. 6c).
Validating blood circulation time and biodistribution data from MSOT by ICP-MS
To validate the quantitative accuracy of MSOT, we employed ICP-MS to measure Au element contents in the blood and organs. The results of ICP-MS were in accord with MSOT's data. Both AuNSs and AuNRs had fast clearance in the bloodstream (half-life less than 3 min). From 3 min to 10 min, the content of AuNSs in the bloodstream dropped from 20.9% ± 1.0% to 15.2% ± 1.3%, while the content of AuNRs dropped from 15.2% ± 0.7% to 7.0% ± 0.9%, which means the blood circulation time of AuNSs was longer than AuNRs with significant difference (P < 0.01) at the first 3 min and 10 min (Fig. 6a). The biodistribution results show that both of gold nanoparticles were mainly captured by reticuloendothelial system and accumulated in the liver and spleen after tail intravenous injection and slightly accumulated in the kidney, heart and lung with low absolute content (Fig. 6b–f). Both AuNSs and AuNRs rapidly accumulated in the liver and spleen at the first 3 min, and then remained stable after 30 min (Fig. 6b and c). There was no significant difference between AuNSs and AuNRs in the liver (Fig. 6b). At the first 3 min, more AuNSs were accumulated in the spleen than AuNRs (Fig. 6c, P < 0.05) in accordance with MSOT results (Fig. 5c and d). Besides, AuNRs had more accumulation in the lung and kidney than AuNSs with significant difference (P < 0.05) which may be attributed to their differences of size and shape (Fig. 6d and f). Although the absolute contents of gold nanoparticles in the heart were very low, there were significant difference (P < 0.05) between AuNSs and AuNRs (Fig. 6e).It is worth nothing that MSOT provides structural and functional information of only one transverse section of a mouse at one time, while ICP-MS provides quantitative elementary information of all the tissues or organs of a mouse at the same time. Thus it is less convenient for MSOT to provide quantitative information of multiple organs or tissues with statistical analysis than ICP-MS. Besides, the limit detection of MSOT (∼3.1 μg mL−1 Au) is much higher than ICP-MS (<0.1 ng mL−1).42 Thus, it is hard to detect the gold nanoparticles in the kidney and heart by MSOT at the 2 mg kg−1 injection dose due to their much lower contents compared to the liver and spleen. However, it is a big challenge for the ICP-MS method to collect samples in such a short time to monitor the early pharmacokinetic changes of drugs or probes with very fast clearance from the bloodstream. ICP-MS also needs to sacrifice animals and requires complicated sample preparation procedures compared to MSOT. Therefore, ICP-MS can be used as the confirmation and supplementary for MSOT, which provides accurate quantitative information with very low detection limit.
Conclusion
Size, shape, surface coating and surface charge are key factors that determine the distribution, translocation, long-term stability and toxicity potential of nanomaterials.10,43,44 Although many studies had been carried out, the relations between these factors were still complicated, which attracted more detailed investigations. In this article, we had prepared chitosan-capped gold nanoparticles in two different shapes (star and rod) and studied their pharmacokinetics and biodistribution in mice by MSOT. Both AuNSs and AuNRs had accurate and strong signal detections by spectral unmixing function of MOST. The intensity of photoacoustic signals of AuNSs was higher than that of AuNRs at the same ODmax. In pharmacokinetic experiments, AuNSs showed longer circulation time in the bloodstream than AuNRs. Both AuNSs and AuNRs experienced rapid clearance in blood-levels followed by a more gradual decrease and had fast and long-time accumulation in the liver and spleen. ICP-MS results verified and supplemented the quantitative results of pharmacokinetic and biodistribution by MSOT, which provides not only the absolute contents of Au element in multiple tissues and organs but also their statistical analysis. The difference between AuNSs and AuNRs was that more AuNSs were accumulated in the spleen than AuNRs at the first 3 min. Besides, AuNRs had more accumulation in the lung and kidney and less accumulation in the heart than AuNSs with significant difference. Although the shape of nanoparticles could affect their in vivo biological effects, this study has shown that both gold nanoparticles in star shape and rod shape undergo similar pharmacokinetic and biodistribution pathway (rapid clearance from the bloodstream and major accumulation in the liver and spleen). Therefore, AuNSs, as a novel photothermal platform, appears to be a potent candidate for diverse biomedical applications as AuNRs does. Finally, our experiments also demonstrate that MSOT can serve as an effective and convenient tool to study gold nanoparticles' pharmacokinetic, biodistribution and specific organs' accumulation and metabolism, especially as a rapid screening method for assessing organ-related distribution and acute toxicity of nanomedicines.Acknowledgements
This work was financially supported by the Ministry of Science and Technology of China (National Basic Research Programs: 2011CB933401 and 2012CB934003), International Science & Technology Cooperation Program of China, Ministry of Science Technology of China (2013DFG32340 and 2014DFG52500), National Major Scientific Instruments Development Project (2011YQ03013406), the National Science Foundation of China (21320102003, 11205166), the National Science Fund for Distinguished Young Scholars (11425520) and State Scholarship Fund of China Scholarship Council (201307970002).References
- M. A. McAteer and R. P. Choudhury, Vasc. Pharmacol., 2013, 58, 31 CrossRef CAS PubMed.
- D. Brambilla, R. Verpillot, B. Le Droumaguet, J. Nicolas, M. Taverna, J. Kona, B. Lettiero, S. H. Hashemi, L. De Kimpe, M. Canovi, M. Gobbi, V. Nicolas, W. Scheper, S. M. Moghimi, I. Tvaroska, P. Couvreur and K. Andrieux, ACS Nano, 2012, 6, 5897 CrossRef CAS PubMed.
- L. Wang, X. Lin, J. Wang, Z. Hu, Y. Ji, S. Hou, Y. Zhao, X. Wu and C. Chen, Adv. Funct. Mater., 2014, 24, 4229 CrossRef CAS.
- Y. Xu, J. Wang, X. Li, Y. Liu, L. Dai, X. Wu and C. Chen, Biomaterials, 2014, 35, 4667 CrossRef CAS PubMed.
- Z. Zhang, J. Wang and C. Chen, Adv. Mater., 2013, 25, 3869 CrossRef CAS PubMed.
- L. G. Xu, Y. Liu, Z. Y. Chen, W. Li, Y. Liu, L. M. Wang, Y. Liu, X. C. Wu, Y. L. Ji, Y. L. Zhao, L. Y. Ma, Y. M. Shao and C. Y. Chen, Nano Lett., 2012, 12, 2003 CrossRef CAS PubMed.
- G. Wu, A. Mikhailovsky, H. A. Khant and J. A. Zasadzinski, Methods Enzymol., 2009, 464, 279 CAS.
- K. Cheng, S. R. Kothapalli, H. Liu, A. L. Koh, J. V. Jokerst, H. Jiang, M. Yang, J. Li, J. Levi, J. C. Wu, S. S. Gambhir and Z. Cheng, J. Am. Chem. Soc., 2014, 136, 3560 CrossRef CAS PubMed.
- Y. Xia, W. Li, C. M. Cobley, J. Chen, X. Xia, Q. Zhang, M. Yang, E. C. Cho and P. K. Brown, Acc. Chem. Res., 2011, 44, 914 CrossRef CAS PubMed.
- E. Boisselier and D. Astruc, Chem. Soc. Rev., 2009, 38, 1759 RSC.
- X. Huang, S. Neretina and M. A. El-Sayed, Adv. Mater., 2009, 21, 4880 CrossRef CAS PubMed.
- Z. Zhang, L. Wang, J. Wang, X. Jiang, X. Li, Z. Hu, Y. Ji, X. Wu and C. Chen, Adv. Mater., 2012, 24, 1418 CrossRef CAS PubMed.
- Z. Zhang, J. Wang, X. Nie, T. Wen, Y. Ji, X. Wu, Y. Zhao and C. Chen, J. Am. Chem. Soc., 2014, 136, 7317 CrossRef CAS PubMed.
- L. Wang, Y.-F. Li, L. Zhou, Y. Liu, L. Meng, K. Zhang, X. Wu, L. Zhang, B. Li and C. Chen, Anal. Bioanal. Chem., 2010, 396, 1105 CrossRef CAS PubMed.
- A. Taruttis, S. Morscher, N. C. Burton, D. Razansky and V. Ntziachristos, PLoS One, 2012, 7, e30941 Search PubMed.
- M. A. Hahn, A. K. Singh, P. Sharma, S. C. Brown and B. M. Moudgil, Anal. Bioanal. Chem., 2011, 399, 3 CrossRef CAS PubMed.
- J. P. Culver, V. Ntziachristos, M. J. Holboke and A. G. Yodh, Opt. Lett., 2001, 26, 701 CrossRef CAS.
- C. Kim, E. C. Cho, J. Chen, K. H. Song, L. Au, C. Favazza, Q. Zhang, C. M. Cobley, F. Gao, Y. Xia and L. V. Wang, ACS Nano, 2010, 4, 4559 CrossRef CAS PubMed.
- S. S. Gambhir, Nat. Rev. Cancer, 2002, 2, 683 CrossRef CAS PubMed.
- D. Kim, S. Park, J. H. Lee, Y. Y. Jeong and S. Jon, J. Am. Chem. Soc., 2007, 129, 7661 CrossRef CAS PubMed.
- J. Kurhanewicz, D. B. Vigneron, R. G. Males, M. G. Swanson, K. K. Yu and H. Hricak, Radiol. Clin., 2000, 38, 115 CrossRef CAS.
- L. V. Wang and S. Hu, Science, 2012, 335, 1458 CrossRef CAS PubMed.
- C. Kim, C. Favazza and L. V. Wang, Chem. Rev., 2010, 110, 2756 CrossRef CAS PubMed.
- L. Nie, S. Wang, X. Wang, P. Rong, A. Bhirde, Y. Ma, G. Liu, P. Huang, G. Lu and X. Chen, Small, 2014, 10, 1585 CrossRef CAS PubMed.
- W. Li, X. Sun, Y. Wang, G. Niu, X. Chen, Z. Qian and L. Nie, Biomed. Opt. Express, 2014, 5, 2679 CrossRef PubMed.
- Y. Qiu, Y. Liu, L. Wang, L. Xu, R. Bai, Y. Ji, X. Wu, Y. Zhao, Y. Li and C. Chen, Biomaterials, 2010, 31, 7606 CrossRef CAS PubMed.
- L. Wang, Y. Liu, W. Li, X. Jiang, Y. Ji, X. Wu, L. Xu, Y. Qiu, K. Zhao, T. Wei, Y. Li, Y. Zhao and C. Chen, Nano Lett., 2011, 11, 772 CrossRef CAS PubMed.
- J. Zhang, X. Nie, Y. Ji, Y. Liu, X. Wu, C. Chen and X. Fang, J. Nanosci. Nanotechnol., 2014, 14, 4124 CrossRef CAS PubMed.
- S. Zhen, F. L. Guo, Y. Li and C. Huang, Sci. China: Chem., 2013, 56, 387 CrossRef CAS.
- W. H. De Jong, W. I. Hagens, P. Krystek, M. C. Burger, A. J. A. M. Sips and R. E. Geertsma, Biomaterials, 2008, 29, 1912 CrossRef CAS PubMed.
- W. Jiang, B. Y. S. Kim, J. T. Rutka and W. C. W. Chan, Nat. Nanotechnol., 2008, 3, 145 CrossRef CAS PubMed.
- Arnida, M. M. Janat-Amsbury, A. Ray, C. M. Peterson and H. Ghandehari, Eur. J. Pharm. Biopharm., 2011, 77, 417 CrossRef CAS PubMed.
- X. Huang, L. Li, T. Liu, N. Hao, H. Liu, D. Chen and F. Tang, ACS Nano, 2011, 5, 5390 CrossRef CAS PubMed.
- H. Yuan, A. M. Fales and T. Vo-Dinh, J. Am. Chem. Soc., 2012, 134, 11358 CrossRef CAS PubMed.
- B. Van de Broek, N. Devoogdt, A. D'Hollander, H.-L. Gijs, K. Jans, L. Lagae, S. Muyldermans, G. Maes and G. Borghs, ACS Nano, 2011, 5, 4319 CrossRef CAS PubMed.
- S. Wang, P. Huang, L. Nie, R. Xing, D. Liu, Z. Wang, J. Lin, S. Chen, G. Niu, G. Lu and X. Chen, Adv. Mater., 2013, 25, 3055 CrossRef CAS PubMed.
- H. Chen, X. Zhang, S. Dai, Y. Ma, S. Cui, S. Achilefu and Y. Gu, Theranostics, 2013, 3, 633 CrossRef CAS PubMed.
- M. L. Tan, P. F. M. Choong and C. R. Dass, J. Pharm. Pharmacol., 2009, 61, 131 CrossRef CAS.
- C. Magnetto, M. Prato, A. Khadjavi, G. Giribaldi, I. Fenoglio, J. Jose, G. R. Gulino, F. Cavallo, E. Quaglino, E. Benintende, G. Varetto, A. Troia, R. Cavalli and C. Guiot, RSC Adv., 2014, 4, 38433 RSC.
- L.-C. Cheng, H. M. Chen, T.-C. Lai, Y.-C. Chan, R.-S. Liu, J. C. Sung, M. Hsiao, C.-H. Chen, L.-J. Her and D. P. Tsai, Nanoscale, 2013, 5, 3931 RSC.
- R. R. Arvizo, O. R. Miranda, D. F. Moyano, C. A. Walden, K. Giri, R. Bhattacharya, J. D. Robertson, V. M. Rotello, J. M. Reid and P. Mukherjee, PLoS One, 2011, 6, e24374 CAS.
- B. Meermann and M. Sperling, Anal. Bioanal. Chem., 2012, 403, 1501 CrossRef CAS PubMed.
- Z. Zhang, J. Wang and C. Chen, Theranostics, 2013, 3, 223 CrossRef CAS PubMed.
- Y. Liu, Y. Zhao, B. Sun and C. Chen, Acc. Chem. Res., 2013, 46, 702 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2015 |