Simultaneous doping and growth of Sn-doped hematite nanocrystalline films with improved photoelectrochemical performance†
Abstract
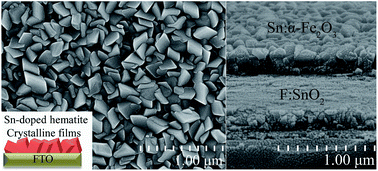
Hematite is an important material used in water splitting and lithium-ion battery electrodes. Electronic conductivity and the visible light-absorption ability of hematite are enhanced by doping impurity ions into the hematite lattice and achieving an appropriate hematite nanostructure. This paper reports the simultaneous doping and growth of tin (Sn)-doped hematite crystalline films on a conducting substrate. The crystalline films were prepared by a hydrothermal process. Laser ablation in liquid induced SnOx colloidal nanoparticles, which were used as the doping source. The obtained compacted films were characterized by scanning electron microscopy, transmission electron microscopy, X-ray diffraction (XRD), X-ray photoelectron spectroscopy, and UV-vis spectrophotometry analyses. XRD results showed the Sn-doped α-Fe2O3 crystalline nanoparticles had dominant active (110) planes. Annealing affected the photoelectrochemical (PEC) performance of the Sn-doped hematite photoanode, and the photocurrent density of the photoanode annealed at 750 °C for 2 h reached the highest value at 0.48 mA cm−2 (at 1.23 V vs. reversible hydrogen electrode). Electrochemical impedance spectroscopy measurements revealed that the charge-transfer resistance of Sn-doped hematite films decreased after the annealing treatment. The improved crystallinity and the preferred (110) plane in the doped crystalline film are favor of the migration of electrons and holes to electrode surfaces, the removal of deleterious surface states and increase of the free electron density, which should contribute to the enhanced PEC performance.

 Please wait while we load your content...
Please wait while we load your content...