A selective fluorescent probe for cysteine and its imaging in live cells†
Youngsam Kima,
Minsuk Choic,
Seokjun Seoa,
Sudesh T. Manjare‡
b,
Sangyong Jonc and
David G. Churchill*a
aMolecular Logic Gate Laboratory, Department of Chemistry, Korea Advanced Institute of Science and Technology (KAIST), 373-1 Guseong-dong, Yuseong-gu, Daejeon, 305-701, Republic of Korea. E-mail: dchurchill@kaist.ac.kr
bCenter for Catalytic Hydrocarbon Functionalizations, Institute for Basic Science (IBS), 373-1 Guseong-dong, Yuseong-gu, Daejeon, 305-701, Republic of Korea
cDepartment of Biological Sciences, Korea Advanced Institute of Science and Technology (KAIST), 373-1 Guseong-dong, Yuseong-gu, Daejeon, 305-701, Republic of Korea
First published on 20th November 2014
Abstract
A probe for the detection of cysteine over homocysteine based on 2-(2′-hydroxyphenyl)benzothiazole (HBT) was prepared and used in confocal microscopy experiments. The probe was designed to block excited state intramolecular proton transfer (ESIPT). When bromopropionyl group protection is removed, HBT is recovered via nucleophilic substitution and intramolecular cyclization. The probe was found to have a detection limit of 2.8 μM and exhibits a ∼20-fold increase. The probe showed cell membrane permeability and efficacy in living Hep3B cells.
Molecular fluorescence and fluorescence microscopy of cellular media continue to be commanding techniques for probing biology.1 In particular, live cell imaging, when connected to small well-defined molecular probes, is a powerful technique in unraveling the concentrations and potential roles of small molecules, proteins and endogenous ions in biology, and help to further link these species to aspects of health and disease.2
Biothiols, such as cysteine (Cys), homocysteine (Hcy) and glutathione (GSH), play critical roles in biological systems. The multiple oxidation states open to sulfur make biothiols valuable for many physiological processes.3 Functions such as detoxification, antioxidant activity, apoptosis, signal transduction and other vital and important biological functions or phenomena are better understood through recent interfacing with novel molecular probes and dyes. However, abnormal concentrations of these thiols can cause various problems and specific damage such as those related to alcoholic cirrhosis, cardiovascular disorders, diabetes mellitus, neurological disorders and stroke.3
Cys, the most reduced type of, and prevalent, biothiol, exists as a monomer present in the active sites of many proteins and protein motifs that function to serve receptor signaling, regulation of gene expression and enzyme regulation. Considering these important roles of Cys, the level of Cys should be high enough to support biological processes.4 Hcy is another sulfur-containing amino acid. The amount of Hcy is controlled via trans-sulfuration and methionine-conserving pathways; it is not obtained from a dietary source. Overproduction of Hcy may give rise to risks and various diseases such as cardiovascular disease, neural tube defects, dementia and Alzheimer's disease.5 GSH is a ubiquitous tripeptide molecule present in the majority of plants and all mammalian tissues as well as in microorganisms. It is widely known to provide redox homeostasis for lipid peroxidation of cellular membranes and other targets (reactive oxygen species (ROS) and reactive nitrogen species (RNS)) found to occur alongside oxidative stress.6
There are various techniques used to detect biothiols in solution media. Even though thiols have similar structure and reactivity, each thiol plays a different biological role. Therefore, it is important to continue to develop and refine novel methods for the detection and discrimination of Cys,7 Hcy,8 and GSH.9 Recently, some fluorescent probes were reported based on specific reactions such as the Michael addition and nucleophilic substitution reactions for the detection of biothiols.10 These organic reaction-based approaches are widely used in chemosensing. Reported probes were designed to position electrophilic α,β-unsaturated groups or halide groups near the ester bond. Once biothiols react via nucleophilic addition or substitution, the nitrogen free amine group undergoes intramolecular cyclization with the proximal ester bond (Fig. 1). Strongin et al. has reported a simultaneous fluorescence probe for the detection of Cys and Hcy from the reaction of 2-(2′-hydroxy-3′-methoxyphenyl)benzothiazole with an α,β-unsaturated carbonyl moiety undergoing a 1,4-Michael addition reaction and tandem intramolecular cyclization.10a Yoon et al. developed a NIR ratiometric based probe for the detection of Cys involving a cyanine framework with an α,β-unsaturated carbonyl moiety that functions in the same manner.10b Chan et al. reported two fluorescein-based probes with one or two α,β-unsaturated carbonyl moieties.10c Kim et al. reported a protected fluorescein involving a bromoacetyl group that undergoes nucleophilic substitution reaction with Cys and intramolecular cyclization.10d Churchill et al. has reported a fluorescein-based probe with chloropropionyl group deprotecting via nucleophilic substitution with Cys and intramolecular cyclization as well.10e Herein, we report a new HBT based probe for the detection of Cys using nucleophilic substitution that involves a bromopropionyl group and subsequent intramolecular cyclization. The bromopropionyl group is expected to show a faster reaction rate than chloropropionyl due to the incorporation of a better leaving group which is more discriminating than the bromopropionyl for the detection of Cys over Hcy because of size differences in ring-formation.
The synthetic procedure of the probe is shown in Scheme 1. 2-(2-Hydroxyphenyl)benzothiazole (HBT) was treated with 2.5 equiv. of bromopropionyl chloride in anhydrous CH2Cl2 at room temperature under nitrogen. The probe was fully characterized by 1H-, 13C-, HMBC-, HSQC- NMR and HR-MS (Fig. S1–S7†). Inspired by reported probes which present and discuss reactions with thiols, the probe was screened with 1 equiv. of various amino acids (Cys, Pro, Thr, Val, Leu, Asn, Trp, Ile, Tyr, Ala, His, Glu, Lys, Met, Asp, Phe) at a concentration of 20 μM (DMSO/10 mM HEPES pH 7.4, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v) and incubated for 10 min. As expected, only Cys showed a dramatic fluorescence change. This modulation exists at ∼467 nm corresponding to the keto-form, and also slightly at ∼380 nm corresponding to the enol-form.11 The fluorescence enhancement with Cys led to a screening with other sulfur-containing amino acids such as Hcy and N-actyl-l-Cys treated under the same conditions. GSH (10 mM, 500 equiv.) was added in the presence of the probe (20 μM), due to its existence at much higher concentrations in cells. The results demonstrated clearly that Cys is selective over other amino acids and even other sulfur-containing amino acids; other sulfur-containing amino acids showed slight fluorescence changes only (Fig. 2).
2 v/v) and incubated for 10 min. As expected, only Cys showed a dramatic fluorescence change. This modulation exists at ∼467 nm corresponding to the keto-form, and also slightly at ∼380 nm corresponding to the enol-form.11 The fluorescence enhancement with Cys led to a screening with other sulfur-containing amino acids such as Hcy and N-actyl-l-Cys treated under the same conditions. GSH (10 mM, 500 equiv.) was added in the presence of the probe (20 μM), due to its existence at much higher concentrations in cells. The results demonstrated clearly that Cys is selective over other amino acids and even other sulfur-containing amino acids; other sulfur-containing amino acids showed slight fluorescence changes only (Fig. 2).
A 20 μM sample of the probe was tested with various concentrations of Cys (0.50 to 2.5 equiv.) and was incubated for 15 min. The concentration of Cys and the fluorescence intensity at ∼467 nm were linearly proportional. The detection limit of Cys was determined to be 2.8 μM (Fig. 2). Compared with the detection limit of probes undergoing nucleophilic substitution process such as the bromoacetate group10d (35 μM) and the chloropropionate group10e (12.8 μM), the present probe has a lower experimental detection limit (2.8 μM). Time-dependent emission spectra of the probe with 1 equiv. of Cys over 1 h were also obtained. It showed a gradual emission intensity increase with time (Fig. S9†). To demonstrate that the bromopropionate group becomes detached and the probe becomes deprotected, the probe was treated and underwent reaction with 1 equiv. of Cys in 3 mL of DMSO: 10 mM HEPES (v/v 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) for 1 h. Then, an aliquot of this reaction mixture was tested and verified by ESI-mass measurements. The spectrum clearly shows that HBT was produced (Fig. S8†). We believe that the recovery of HBT is due to nucleophilic substitution and intramolecular cyclization (Scheme 2).10d,e This tandem reaction mechanism indicates the difference of fluorescence enhancement between Cys and Hcy is based on a kinetically favored ring-formation. As has been found in previous work, the seven-membered lactam ring formation with Cys is favored more than the eight-membered lactam formation with Hcy concentration arising from the intramolecular cyclization step.10a
2) for 1 h. Then, an aliquot of this reaction mixture was tested and verified by ESI-mass measurements. The spectrum clearly shows that HBT was produced (Fig. S8†). We believe that the recovery of HBT is due to nucleophilic substitution and intramolecular cyclization (Scheme 2).10d,e This tandem reaction mechanism indicates the difference of fluorescence enhancement between Cys and Hcy is based on a kinetically favored ring-formation. As has been found in previous work, the seven-membered lactam ring formation with Cys is favored more than the eight-membered lactam formation with Hcy concentration arising from the intramolecular cyclization step.10a
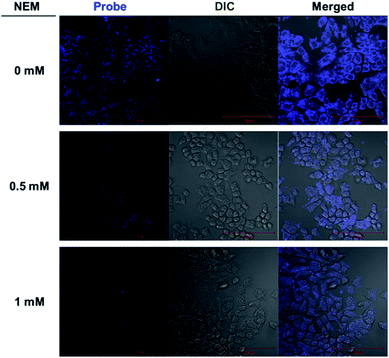
In order to further demonstrate the permeability of the probe and its worthiness to be considered as a real potential detection platform for use in living cells, various confocal microscopy experiments were performed. When Hep3B cells were incubated with various concentrations of the probe (20 μM), a strong blue fluorescence signal was exhibited in the cytoplasm (Fig. S10†). This result indicates the cell-permeability of the probe (transduction the cellular membrane). To help confirm the emission from the Hep3B cells is caused by the reaction of the probe with intracellular thiol species and not other related species, Hep3B cells were pre-treated with 0.5 mM and 1 mM of N-ethylmaleimide (NEM), a thiol-specific reagent,12 followed by treatment with probe. We treated cells with various concentrations of NEM. Cells were incubated for 1 h and washed three times with DPBS, followed by treatment with the probe (20 μM) for 10 min. The fluorescence intensity decreased as the concentration of NEM was increased. These results suggested that the probe reacts specifically with thiol in living cells (Fig. 3).
Conclusions
In conclusion, herein, we have developed and fully characterized a new probe based on the reliable ESIPT system for selective detection of Cys over other amino acids. The probe generates HBT via nucleophilic substitution and intramolecular cyclization reaction. Based on kinetic intramolecular cyclization, Cys is discriminated well beyond that of other sulfur-containing amino acids. The detection limit was found to be 2.8 μM. Furthermore, the probe was also demonstrated for the detection of thiols in Hep3B cells through the use of confocal microscopy imaging.Acknowledgements
The Molecular Logic Gate Laboratory operated by D.G.C. acknowledges financial support from the NRF (National Research Foundation) of Korea (2011-0017280) and the “End Run” commercialization (N01140684). S.T.M. acknowledges support from Institute of Basic Science (IBS), Korea.Notes and references
- (a) S. Watanabe and E. M. Jorgensen, Methods Cell Biol., 2012, 111, 283 CrossRef CAS PubMed; (b) V. Magidson and A. Khodjakov, Methods Cell Biol., 2013, 114, 545 CrossRef PubMed.
- (a) T. Haraguchi, Cell Struct. Funct., 2002, 27, 333 CrossRef CAS; (b) M. M. Frigault, J. Lacoste, J. L. Swift and C. M. Brown, J. Cell Sci., 2009, 122, 753 CrossRef CAS PubMed; (c) K. Nienhaus and G. U. Nienhaus, Chem. Soc. Rev., 2014, 43, 1088 RSC.
- M. Prakash, M. S. Shetty, P. Tilak and N. Anwar, J. Health Allied Sci., 2009, 8, 1 Search PubMed.
- (a) W. Droge and E. Holm, FASEB J., 1997, 11, 1077 CAS; (b) S. A. Lipton, Y.-B. Choi, H. Takahashi, D. Zhang, W. Li, A. Godzik and L. A. Bankston, Trends Neurosci., 2002, 25, 474 CrossRef CAS; (c) D. Barford, Curr. Opin. Struct. Biol., 2004, 14, 679 CrossRef CAS PubMed; (d) D. Brömme and S. Wilson, Extracellular Matrix Degradation, ed. R. P. Mecham, Berlin, 2011, p. 23 Search PubMed; (e) N. Gould, P. T. Doulias, M. Tenopoulou, K. Raju and H. Ischiropoulos, J. Biol. Chem., 2013, 288, 26473 CrossRef CAS PubMed.
- (a) M. L. Urbanowski and G. V. Stauffer, J. Bacteriol., 1989, 171, 3277 CAS; (b) J. Selhub, Annu. Rev. Nutr., 1999, 19, 217 CrossRef CAS PubMed; (c) P. I. Ho, S. C. Collins, S. Dhitavat, D. Ortiz, D. Ashline, E. Rogers and T. B. Shea, J. Neurochem., 2001, 78, 1 CrossRef; (d) A. S. Wierzbicki, Diab. Vasc. Dis. Res., 2007, 4, 143 CrossRef PubMed; (e) S. M. Krishna, A. Dear, J. M. Craig, P. E. Norman and J. Golledge, Atherosclerosis, 2013, 228, 295 CrossRef CAS PubMed.
- (a) C. Kerksick and D. Willoughby, J. Int. Soc. Sports Nutr., 2005, 2, 38 CrossRef PubMed; (b) S. Chakravarthi, C. E. Jessop and N. J. Bulleid, EMBO Rep., 2006, 7, 271 CrossRef CAS PubMed; (c) V. I. Lushchak, J. Amino Acids, 2012, 2012, 736837 Search PubMed.
- (a) L. Yuan, W. Lin and Y. Yang, Chem. Commun., 2011, 47, 6275 RSC; (b) X. Zhou, X. Jin, G. Sun, D. Li and X. Wu, Chem. Commun., 2012, 48, 8793 RSC; (c) X. Zhou, X. Jin, G. Sun and X. Wu, Chem.–Eur. J., 2013, 19, 7817 CrossRef CAS PubMed; (d) H. S. Jung, K. C. Ko, G. Kim, A. Lee, Y. Na, C. Kang, J. Y. Lee and J. S. Kim, Org. Lett., 2011, 13, 1498 CrossRef CAS PubMed; (e) M. Isik, T. Ozdemir, I. S. Turan, S. Kolemen and E. U. Akkaya, Org. Lett., 2013, 15, 216 CrossRef CAS PubMed.
- (a) H. Y. Lee, Y. P. Choi, S. Kim, T. Yoon, Z. Guo, S. Lee, K. M. K. Swamy, G. Kim, J. Y. Lee, I. Shin and J. Yoon, Chem. Commun., 2014, 50, 6967 RSC; (b) A. Barve, M. Lowry, J. O. Escobedo, K. T. Huynh, L. Hakuna and R. M. Strongin, Chem. Commun., 2014, 50, 8219 RSC; (c) J. Zhang, X. Jiang, X. Shao, J. Zhao, Y. Su, D. Xi, H. Yu, S. Yue, L. Xiao and W. Zhao, RSC Adv., 2014, 4, 54080 RSC.
- (a) X. Yang, Q. Huang, Y. Zhong, Z. Li, H. Li, M. Lowry, J. O. Escobedo and R. M. Strongin, Chem. Sci., 2014, 5, 2177 RSC; (b) J. Yin, Y. Kwon, D. Kim, D. Lee, G. Kim, Y. Hu, J. Ryu and J. Yoon, J. Am. Chem. Soc., 2014, 136, 5351 CrossRef CAS PubMed; (c) S. Lim, K. Hong, D. I. Kim, H. Kwon and H. Kim, J. Am. Chem. Soc., 2014, 136, 7018 CrossRef CAS PubMed; (d) S. Wang, Q. Wu, H. Wang, X. Zheng, S. Shen, Y. Zhang, J. Miao and B. Zhao, Analyst, 2013, 138, 7169 RSC; (e) S. Wang, Q. Wu, H. Wang, X. Zheng, S. Shen, Y. Zhang, J. Miao and B. Zhao, Biosens. Bioelectron., 2014, 55, 386 CrossRef CAS PubMed.
- (a) X. Yang, Y. Guo and R. M. Strongin, Angew. Chem., Int. Ed., 2011, 50, 10690 CrossRef CAS PubMed; (b) Z. Guo, S. Nam, S. Park and J. Yoon, Chem. Sci., 2012, 3, 2760 RSC; (c) H. Wang, G. Zhou, H. Gai and X. Chen, Chem. Commun., 2012, 48, 8341 RSC; (d) K.-H. Hong, S.-Y. Lim, M.-Y. Yun, J.-W. Lim, J.-H. Woo, H. Kwon and H.-J. Kim, Tetrahedron Lett., 2013, 54, 3003 CrossRef CAS PubMed; (e) D. P. Murale, H. Kim, W. S. Choi and D. G. Churchill, RSC Adv., 2014, 4, 5289 RSC; (f) D. P. Murale, H. Kim, W. S. Choi, Y. Kim and D. G. Churchill, RSC Adv., 2014, 4, 46513 RSC.
- S. M. Aly, A. Usman, M. Alzayer, G. A. Hamdi, E. Alarousu and O. F. Mohammad, J. Phys. Chem. B, 2014 DOI:10.1021/jp508777h.
- J. D. Grecor, J. Am. Chem. Soc., 1955, 77, 3922 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra12981d |
| ‡ Current address: Assistant Professor, Department of Chemistry, University of Mumbai, Vidyanagari, Santacruz (East), Mumbai-400098, India. |
| This journal is © The Royal Society of Chemistry 2014 |