A low-cost method for obtaining high-value bio-based propylene glycol from sugar beet pulp
J. Berlowska*a,
M. Binczarskib,
M. Dudkiewicza,
H. Kalinowskac,
I. A. Witonskab and
A. V. Stanishevskyd
aInstitute of Fermentation Technology and Microbiology, Lodz University of Technology, Wolczanska 171/173, 90-924 Lodz, Poland. E-mail: joanna.berlowska@p.lodz.pl
bInstitute of General and Ecological Chemistry, Lodz University of Technology, Zeromskiego 116, 90-924, Lodz, Poland
cInstitute of Technical Biochemistry, Lodz University of Technology, Stefanowskiego 4/10, 90-924 Lodz, Poland
dDepartment of Physics, University of Alabama at Birmingham, Birmingham, AL 35294, USA
First published on 2nd December 2014
Abstract
A new low-cost pathway for the production of high-value propylene glycol (PG) is proposed. This route of waste biomass utilization employs catalytic reduction of lactic acid obtained from fermented enzymatic digests of sugar beet pulp.
Propylene glycol (PG, 1,2-propanediol) is one of the most important raw chemical materials in the world.1,2 Desirable properties such as viscosity, no odour, negligible toxicity and a low freezing point mean that 1,2-propanediol is in great demand from many branches of industry,3–6 including the food, pharmaceutical and cosmetics industries. However, since industry uses PG on a very large scale (2.81 million tonnes in 2013, increasing by around 8% each year),6 the sustainability of traditional production is threatened by the cost and future availability of crude oil, as well as by environmental concerns. Most world production of propylene glycol employs non-catalytic, high-temperature and high-pressure hydrolysis of propylene oxide, which is obtained from propylene.
In response to the need for new pathways for the production of PG using renewable raw materials, a more recent method was developed which uses hydrogenolysis of glycerol derived mainly from soybeans and rapeseed.7 This process can be performed successfully under mild conditions (around 1.5 MPa, 200 °C) using nickel, palladium, platinum, copper and copper-chromite catalysts.8–12 However, it is not without disadvantages, most notably the high cost involved, which if this method were employed on a large scale would lead to increased food prices on world markets.
Another way of obtaining PG is through the reduction of lactic acid (LA).13–17 LA can be obtained easily by fermentation of saccharide feed,15,18,19 and is an extremely useful building block in the synthesis of chemicals such as acrylic acid, pyruvic acid, 2,3-pentanedione, lactic acid esters and propylene glycol.20 Hydrogenation of LA into PG has been performed over supported metallic catalysts, mainly based on ruthenium,16 rhenium,21 platinum,22 nickel22 or copper,22 under mild conditions (0.1–14.5 MPa, 70–240 °C).13–16,21,22 This pathway for the transformation of LA into PG is of great interest to industry.4,23–26
Researchers from Lodz University of Technology, in cooperation with the Polish National Sugar Company Ltd., have developed a new method of propylene glycol synthesis via catalytic hydrogenation of lactic acid obtained by the fermentation of sugar beet pulp hydrolyzates (Fig. 1). The cost of raw materials is one of the key factors that determine the economic viability of fermentation processes. Pure glucose, sucrose, starch, etc. are expensive feedstocks for lactic acid production. Their replacement with inexpensive substrates, such as dairy byproducts, food and industrial wastes or agricultural residues (lignocellulose/hemicellulose hydrolyzates, cottonseed hulls, corn cob, corn stalks, sugarcane pressmud, cassava bagasse, cellulose, carrot processing waste, corn fiber hydrolysates and wheat bran) and glycerol, promises to cut the costs of lactic acid production.27–30 Moreover, finding economical and environmentally-friendly uses for byproducts of food processing furthers the aims of sustainable development in the food industry.
The choice of substrate is usually a question of geographic availability. An abundant but underexploited residue in Poland is sugar beet pulp (SBP), which remains after sucrose extraction. To date, SBP has been used mainly as animal feed or, in regions with no livestock farming, sent to landfill. Its low dry-matter (18–23% w/w) content renders beet residue unsuitable for use as a fuel in heat and power production. However, its chemical composition makes it a promising candidate for bio-production.31 Its qualities include a very low lignin level (around 2% of dry matter) and high carbohydrate content (75% w/w of dry matter), including pectin (24–32%), cellulose (22–30%) and hemicellulose (22–30%).32,33 Its principal components can be converted into hexose and pentose feedstocks for use in various fermentation processes.31,34
Enzymatic saccharification of this complex material requires the concerted action of cellulases, hemicellulases (including arabinosidases) and pectinases.35 In our research, a mixture of two commercial multienzyme preparations, Viscozyme and Ultraflo Max from Novozymes, containing these polysaccharidases (Table 1), was found to efficiently saccharify polysaccharides contained in the sugar beet pulp, which was simply suspended in warm water to achieve a dry matter concentration of around 10% (w/v). The conditions of saccharification were optimized at the laboratory scale and the composition of the resulting hydrolysates was determined using chemical, enzymatic and HPLC methods. After 24 hours of hydrolysis, conducted at 50 °C, glucose yields in the hydrolysates were over 30% of the initial sugar beet pulp dry matter. The dominant reducing sugars released during the process were glucose (around 30% of the total reducing sugars), arabinose and galacturonic acid (both around 25%), while xylose accounted for around 2% of all monosaccharides. Concentrations of fermentation inhibitors, such as furfural and phenolic acids, were below 1 μg mL−1.
| Enzyme | Activity (U mL−1) | |||
|---|---|---|---|---|
| Cellulases | Invertase | Xylanases | Pectinases | |
| Viscozyme | 22.6 | 63.3 | 26.3 | 174.6 |
| Ultraflo Max | 41.8 | 0.3 | 53.3 | 33.3 |
Sugar industry by-products have recently received considerable attention as possible feedstocks in the production of value-added products.31 Studies have focused mainly on the production of monosaccharides for use in bioethanol or biogas synthesis from beet waste biomass.35 Zheng15 reports lactic acid yield, carbohydrate loss (cellulose and hemicellulose) and lignin removal during ensilage of sugar beet pulp. However, until now only molasses had been used to produce lactic acid.19 Our research suggests that SBP hydrolysate may be a particularly suitable feedstock for microbial lactic acid fermentation.
Lactic acid bacteria (LABs) have complex nutritional requirements, due to their limited biosynthesis capacity.36,37 Agro-industrial residues, which are rich in carbohydrates, are of limited use due to their low protein content.27 The use for LAB cultivation of media derived from plant materials has been considered in many combinations, including with nitrogen and vitamin supplements. Enrichment with nutrients, especially with MRS growth medium ingredients (mainly yeast extract, peptone and meat extract), has in general a positive effect on LA production efficiency.37 The medium obtained in our experiments via hydrolysis was used only as a carbon source, while non-sugar components of MRS broth provided minerals and nutritional ingredients.
The monosaccharide profile of sugar beet hydrolysates was analyzed using high performance liquid chromatography, performed on Waters 600S equipped with a Waters 717 autosampler and a Gilson PrepELS II Light Scattering Detector. Water was used as the mobile phase Ca2+ and sulfonated styrene–divinylbenzene as the stationary phases in a Rezex™ column. The liquid contained around 5.5% (w/v) total reducing saccharides, including glucose – 1.3%, galactose – 0.7%, xylose – 0.55%, rhamnose – 0.5%, arabinose – 0.65%, raffinose – 1.4%, and fructose – 0.3%.
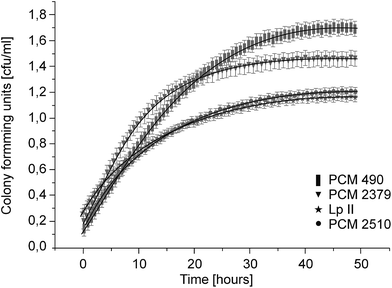
Three collection strains (Polish Collection of Microorganisms) were used in the study: Lactococcus lactis 2379, Lactobacillus acidophilus 2510 and Lactobacillus delbrueckii 490, as well as the environmental isolate (from sugar beet pulp) Lactobacillus plantarum II. Aerobic cultivation of lactic acid bacteria was conducted at 37 °C for 48 hours. The growth of bacteria was measured via spectrophotometric (optical density measurements) and plate count methods. Lactic acid production was measured by spectrophotometry using a D-/L-lactic acid assay kit (megazyme). All of the strains tested reached the stationary growth phase after around 30 hours of cultivation (Fig. 2). However, the sugar utilization profiles and acidification dynamics were found to be strain-dependent (Table 2 and Fig. 3). In most cases, lactic acid bacteria such as L. delbrueckii, L. helveticus and L. acidophilus, are able to convert cellulose-derived glucose, but not hemicellulose-derived sugars, into lactic acid. Only certain strains, L. pentosus, L. bifermentans, L. plantarum, L. brevis, L. delbrueckii, Leuconostoc lactis and Lacctoccocus lactis, are also capable of converting xylose into lactic acid and acetic acid effectively through heterolactic fermentation.19,20,22 However, L. lactis PCM 2379 did not utilize pentoses in our studies. According to Hofvendahl's juxtaposition, sugars such as xylose, galactose, arabinose, lactose, fructose and hydrolyzed cellulose are less effective substrates in fermentation processes than glucose.37 The sequential uptake of various carbohydrates may, however, result in reduced product yields. A few LAB strains are reported to consume lignocelluloses derived sugars simultaneously.28,29 To obtain the required lactic acid yields from sugar beet pulp hydrolyzate, mixed starter cultures could be used as well as sequential inoculation with a strain which has different fermentation abilities. In our study, complete consumption of glucose and fructose and partial utilization of galactose (Lactobacillus strains) and arabinose (L. delbrueckii and L. plantarum) was observed with all four strains. The strains which showed the ability to assimilate the widest range of carbon sources were isolated from the sugar beet pulp, which had been subjected to enzymatic hydrolysis.
| Microorganism | CFU/mL | Sugar utilization [%] | Concentration of lactic acid [g L−1] | Yield of lactic acid produced [g] to substrate consumed [g] | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Glucose | Fructose | Xylose | Rhamnose | Raffinose | Galactose | Arabinose | ||||
| L. lactis PCM 2379 | 8.27 × 108 | 99.13 | 98.29 | 0 | 3.57 | 19.02 | 0 | 0 | 8.18 | 0.45 |
| L. acidophilus PCM 2510 | 7.95 × 108 | 98.90 | 98.92 | 2.16 | 0 | 0 | 39.31 | 7.26 | 7.82 | 0.39 |
| L. delbrueckii PCM 490 | 5.48 × 108 | 99.97 | 99.06 | 6.06 | 0 | 5.08 | 43.75 | 13.51 | 8.47 | 0.39 |
| L. plantarum II | 2.89 × 108 | 99.19 | 98.61 | 13.66 | 0 | 0 | 38.23 | 26.34 | 8.40 | 0.42 |
 | ||
| Fig. 3 Relationship between growth and lactic acid production by the 4 tested strains of lactic acid bacteria. | ||
In such complex media as biomass hydrolysates, the presence of inhibitory substances can cause the elongation of the adaptive phase (Fig. 3). Another barrier to using lignocellulosic derivatives as fermentation media is that the sugar composition of this biomass is inherently heterogeneous.38 Moreover, in many lactic acid fermentations, mixed carbohydrate utilization is achieved sequentially, and glucose catabolite repression occurs.28,29 Abdel-Rahman reports lactic acid production (g/g substrate consumed) from lignocellulosic biomass materials and lignocellulose-derived sugars by lactic acid bacteria in a range from 0.18 for L. delbreuckii NRRL-B445 cultivated on cellulose to 0.99 for L. delbrueckii UFV H2B20 grown on brewer's spent grain.28,29 In our study, medium level product yields were achieved of 0.39–0.45 g LA per g fermentable carbohydrates. However, the fermentation processes were carried out without the chemical removal of lactic acid and product inhibition is thought to have occurred.
In further investigations, biologically obtained lactic acid was used as a substrate. After the biological process and biomass separation, the supernatants were purified on a mixture of active carbon and silica and then treated. Lactic acid purification is one of the most costly steps in the production process.28 In the most common procedures, the fermentation medium is neutralized using calcium carbonate. The media containing calcium lactate is filtered, carbon treated, evaporated and acidified using sulfuric acid. Hydrolysis, esterification and distillation are then conducted to obtain pure acid. Drawbacks of this method include the large amounts of sulfuric acid and calcium sulfate that are, respectively, used and generated as a by-product.39
Moreover, impurities contained in the final product must be removed, involving additional steps such as extraction, ion exchange, membrane separation or electrodialysis.13,15,19 Due to the high cost of purifying media post fermentation, we also studied the effect of impurities on PG formation over 5%Ru/C catalyst. The postfermentation medium for L. delbrueckii PCM 490 was catalytically reduced as an untreated sample, as well as the samples which had undergone purification on active carbon (ERCARBON GE, 3 g 50 mL−1) or on a mixture of active carbon and silica (POCH Gliwice SA, 5 g 50 mL−1) (Table 3).
| Substrate | C0LA [mol dm−3] | Conversion of LA [%] | Selectivity to PG [%] |
|---|---|---|---|
| a 50 mL of fermentation broth after purification on 3 g of Cact.b 50 mL of fermentation broth after purification of on the mixture of 3 g of Cact and 5 g SiO2. | |||
| Water solution lactic acid (CHEMPUR) | 0.500 | 97.4 | 62.4 |
| Postfermentation broth L. delbrueckii PCM 490 | 0.094 | 2.4 | 0 |
| Postfermentation broth L. delbrueckii PCM 490a | 0.081 | 18.3 | 67.9 |
| Postfermentation broth L. delbrueckii PCM 490b | 0.085 | 90.8 | 61.7 |
| Postfermentation broth L. lactis PCM 2379b | 0.080 | 91.1 | 81.1 |
| Postfermentation broth L. acidphilus 2510b | 0.078 | 92.3 | 64.2 |
Hydrogenation of LA was performed in a 50 mL autoclave (Parr Company) at a temperature of 130 °C and under 3.5 MPa of H2 pressure. The reactions were conducted with equal amounts of catalyst (mcat = 0.5 g). The mixture was stirred at 500 rpm. The autoclave was flushed with Ar, then flushed again with H2, and pressurized with H2 to 3.5 MPa. The temperature was gradually raised to 130 °C at a heating rate of 20 °C min−1. The reaction was sustained for 4 hours. The reaction conditions were optimized for 5%Ru/C catalyst (Sigma-Aldrich, CAS 206180) (Fig. 4). After the reaction, the autoclave was cooled to room temperature and the reaction mixture filtered and analyzed using an HPLC (LaChrome, Merck-Hitachi with UV detector) to determine the concentration of lactic acid. Products of LA hydrogenation were also screened for using GC-FID analysis (Hewlet Packard 5890A with FID detector). The liquid products were also analyzed using a PerkinElmer GC-MS (model Clarus 580 with MS Clarus SQ 8 S).
The results of catalytic reduction of all fermentation media which had undergone partial purification are summarized in Table 3. On the basis of these results, it was concluded that this treatment of the broths was sufficient for the effective conversion of LA into PG. The proposed method of partial purification of fermentation broths allows the problematic and expensive steps of lactic acid purification and byproduct utilization to be avoided, which is particularly important for industrial applications.
Conclusions
A new, bio-catalytic method of propylene glycol production from enzymatic digests of sugar beet pulp enables the replacement of fossil resources with byproducts from food processing. Appropriate strains of lactic acid bacteria efficiently convert sugars contained in enzymatic hydrolysates of SBP into lactate, which is reduced to propylene glycol via heterogenic catalysis.Acknowledgements
The authors would like to acknowledge the contribution of the National Centre for Research and Development, Applied Research Programme – Project PBS1/B8/3/2012.Notes and references
- J. J. Bozell and G. R. Petersen, Green Chem., 2010, 12, 539 RSC.
- P. N. R. Vennestrøm, C. M. Osmundsen, C. H. Christensen and E. Taarning, Angew. Chem., 2011, 50(45), 10502 CrossRef PubMed.
- A. E. Martin and F. H. Murphy, Glycols, Propylene glycols, Dow Chemicals, report#117-01785-0306, 2014.
- Z. Zhang, D. J. Miller and J. E. Jackson, US Patent 6,403,844 B1, 2002.
- T. A. Werpy, J. G. Fryye, A. H. Zacher and D. J. Miller, US Patent 7,038,094 B2, 2006.
- Merchant Research & Consulting Ltd., World Propylene Glycol Market, 2014, http://mcgroup.co.uk/news/20140418/propylene-glycol-market-reach-supplydemand-balance-2015.html.
- M. Pagliaro and M. Rossi, The future of glycerol, RSC Green Chemistry No 8, ISBN: 978-1-84973-046-4, The Royal Society of Chemistry, Cambridge, UK, 2010 Search PubMed.
- M. A. Dasari, P.-P. Kiatsimkul, W. R. Sutterlin and G. J. Suppes, Appl. Catal., A, 2005, 281(1–2), 225–231 CrossRef CAS PubMed.
- J. Feng, H. Fu, J. Wang, R. Li, H. Chen and X. Li, Catal. Commun., 2008, 9(6), 1458 CrossRef CAS PubMed.
- M. G. Musolino, L. A. Scarpino, F. Mauriello and R. Pietropaolo, Green Chem., 2009, 11, 1511 RSC.
- E. S. Vasiliadou, T. M. Eggenhuisen, P. Munnik, P. E. de Jongh, K. P. de Jong and A. A. Lemonidou, Appl. Catal., B, 2014, 145, 108 CrossRef CAS PubMed.
- S. Zhau, X. Gao, Y. Zhau, Y. Zhau, H. Zheng and Y. Li, J. Catal., 2013, 303, 70 CrossRef PubMed.
- P. Mäki-Arvela, I. L. Simakowa, T. Salmi and D. Yu. Murzin, Chem. Rev., 2014, 114, 1909 CrossRef PubMed.
- S. Varadarajan and D. J. Miller, Biotechnol. Prog., 1999, 15, 845 CrossRef CAS PubMed.
- M. Dusselier, P. Van Wouwe, A. Dewaele, E. Makshina and B. F. Sels, Energy Environ. Sci., 2013, 6, 1415 CAS.
- H. Jang, S.-H. Kim, D. Lee, S. E. Shim, S.-H. Baeck, B. S. Kim and T. S. Chang, J. Mol. Catal. A: Chem., 2013, 380, 57 CrossRef CAS PubMed.
- Z. Zhang, J. Miller, J. E. Jackson, US Patent 6,403,844 B1, 2002.
- Z. Zhang, J. E. Jackson and D. J. Miller, Bioresour. Technol., 2008, 99, 5873 CrossRef CAS PubMed.
- R. Datta and M. Henry, J. Chem. Technol. Biotechnol., 2006, 81, 1119 CrossRef CAS.
- P. Gallezot, Chem. Soc. Rev., 2012, 41, 1538 RSC.
- H. Broadbent, G. Campbell, W. Bartley and J. Johanson, J. Org. Chem., 1959, 24, 1847 CrossRef CAS.
- T. Y. Jang, K. B. Chung, H. R. Eom, D. K. Noch, I. K. Song, J. Yi and S. Baeck, Res. Chem. Intermed., 2011, 37, 1275 CrossRef CAS.
- M. Kitson and P. S. Williams, US Patent 5149680 A, 1992.
- M. Kitson and P. S. Williams, US Patent 4985572 A, 1991.
- T. A. Werpy, J. G. Frye Jr, A. H. Zacher and D. J. Miller, US Patent 6841085 B2, 2005.
- T. Werpy, J. G. Frye Jr, Y. Wang and A. H. Zacher, US 7186668 B2, 2007.
- R. P. John, G. S. Anisha, K. M. Nampoothiri and A. Pandey, Biotechnol. Adv., 2009, 27, 145 CrossRef CAS PubMed.
- M. A. Abdel-Rahman, Y. Tashiro and K. Sonomoto, J. Biotechnol., 2011, 156, 286 CrossRef CAS PubMed.
- M. A. Abdel-Rahman, Y. Tashiro and K. Sonomoto, Biotechnol. Adv., 2013, 31(6), 877 CrossRef CAS PubMed.
- X. Jiang, Y. Xue, A. Wang, L. Wang, G. Zhang, Q. Zeng, B. Yu and Y. Ma, Bioresour. Technol., 2013, 143, 665 CrossRef CAS PubMed.
- S. Kühnel, H. A. Schols and H. Gruppen, Biotechnol. Biofuels, 2011, 4, 14 CrossRef PubMed.
- Y. Zheng, C. Yu, Y.-S. Cheng, R. Zhang, B. Jenkins and J. S. Vander Gheynst, Bioresour. Technol., 2011, 102, 1489 CrossRef CAS PubMed.
- Y. Zheng, C. Yu, Y.-S. Cheng, C. Lee, C. W. Simmons, T. M. Dooley, R. Zhang, B. M. Jenkins and J. S. Vander Gheynst, Appl. Energy, 2012, 93, 168 CrossRef CAS PubMed.
- K. Zieminski, I. Romanowska, M. Kowalska-Wentel and M. Cyran, Bioresour. Technol., 2014, 166, 187 CrossRef CAS PubMed.
- A. G. M. Leijdekkers, J. P. M. Bink, S. Geutjes, H. A. Schols and H. Gruppen, Bioresour. Technol., 2013, 128, 518 CrossRef CAS PubMed.
- F. A. C. Martinez, E. M. Balciunas, J. M. Salgado, J. M. D. Gonzalez, A. Converti and R. Pinheiro de Souza Oliveira, Trends Food Sci. Technol., 2013, 30, 70 CrossRef CAS PubMed.
- K. Hofvendahl and B. Hahn-Hagerdal, Enzyme Microb. Technol., 2000, 26, 87 CrossRef CAS.
- J.-H. Kim, D. E. Block, S. P. Shoemaker and D. A. Mills, Appl. Microbiol. Biotechnol., 2010, 86, 1375 CrossRef CAS PubMed.
- Y. Li and F. Cui, in Sustainable Biotechnology: Sources of Renewable Energy, Springer Science + Business Media B.V., Dortrecht Heidelberg London New York, 2010, p. 211 Search PubMed.
| This journal is © The Royal Society of Chemistry 2015 |