TBP induced double cloud point in aqueous EO13PO30EO13 solutions: investigating the evolution of associated micellar characteristics as a function of temperature
Abstract
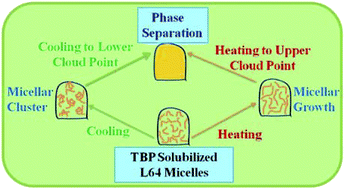
Pluronics are considered as potential materials for the removal of tributyl phosphate (TBP) from nuclear fuel reprocessing plants. Recent studies showed that solubilization of TBP in aqueous solution of Pluronic L64 reduces its cloud point (CP) and in addition, induces a second CP at low temperature. In this manuscript we have attempted to understand the origin of this lower CP (LCP) by carrying out DLS, SANS, viscosity and fluorescence measurement studies on 10% aqueous L64 solutions containing 60 mM TBP. The studies show that on approaching both LCP (≈25 °C) and the conventional or upper CP (UCP ≈ 42.5 °C), the copolymer solution exhibits a large increase in light scattering intensity and apparent micellar radius. Detailed studies suggest that whereas the copolymer micelles undergo a progressive growth on heating to the UCP, they form a micellar cluster upon cooling down to the LCP due to the onset of an inter-micellar attractive interaction. Although such clusters are known to form in micellar solutions of non ionic surfactants on approaching the UCP, their formation upon cooling is the first of its kind for them. The observed unusual behavior has possibly been triggered by dehydration of micellar corona caused by relocation of TBP from micellar core to corona upon cooling down to the LCP.

 Please wait while we load your content...
Please wait while we load your content...