Total synthesis of MECA-79†
Abstract
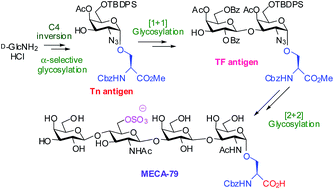
The MECA-79 antigen is a sulfated mucin type core-1 extended O-glycan which is a potential anti-inflammatory agent. Herein we report a total synthesis of MECA-79 via a convergent [2 + 2] glycosylation route. The synthesis relies on efficient transformation of D-glucosamine into the orthogonally protected Tn antigen derivative and its elaboration into the TF antigen en route to MECA-79.

 Please wait while we load your content...
Please wait while we load your content...