Pumpkin stem-derived activated carbons as counter electrodes for dye-sensitized solar cells†
Rajesh Madhu‡
a,
Vediyappan Veeramani‡a,
Shen-Ming Chen*a,
Jayabal Palanisamyb and
A. T. Ezhil Viliana
aElectroanalysis and Bioelectrochemistry Lab, Department of Chemical Engineering and Biotechnology, National Taipei University of Technology, Taipei 10608, Taiwan. E-mail: smchen78@ms15.hinet.net
bDepartment of Laser Studies, School of Physics, Madurai Kamaraj University, Madurai-625021, India
First published on 14th November 2014
Abstract
Activated carbons (ACs) with a high surface area have been prepared from pumpkin stem wastes, using a simple and eco-friendly method. The as-prepared samples were characterized by field-emission scanning electron microscopy, high-resolution transmission electron microscopy, CHNS elemental analysis, and Raman spectroscopy. Furthermore, the AC samples were coated on indium tin oxide (ITO) substrates by the doctor blade technique and were used as counter electrodes (CEs) for dye sensitized solar cells (DSSCs). The fabricated DSSCs with AC counter electrodes showed a high power conversion efficiency of 2.79%. Notably, the excellent performance of the dye-sensitized solar cells fabricated with AC modified CEs surpassed that of several carbon-based counter electrodes in the literature.
Dye-sensitized photoelectrochemical solar cells (DSSCs) have gained much attention from researchers over recent decades due to their sensible power conversion efficiency (PCE), low cost and easy fabrication. The DSSCs consist of (1) transparent conductive oxides, (2) semiconductor oxide film, (3) a sensitizer adsorbed onto the surface of the semiconductor, (4) an electrolyte containing a redox mediator, and (5) a counter electrode (CE).1,2 Among the constituent components in DSSCs, the counter electrode serves an important role in transferring electrons from external circuits to redox electrolytes and catalyzing the reduction of the triiodide ion (I3−). Generally, platinum overloaded on conducting glass is used as a counter electrode in DSSCs for the reduction of triiodide in the redox electrolyte. Although Pt has very high catalytic activity and stability, it is the one of the most expensive components in DSSCs. As an alternative approach to overcome this problem, it is desirable to produce new CEs. Recently, carbon based CEs have played a crucial role in DSSCs and are quite attractive to replace the Pt CEs due to their low cost production, large surface area, high electronic conductivity, corrosion resistance towards I2 and chemical stability. There are several ranges of carbon materials such as carbon nanotubes, carbon black, activated carbon, graphite and graphene which have been employed as counter electrodes.3–5 Among them, activated carbons (ACs) are readily available, low cost, and their preparation procedure from biomass is simple and environmentally friendly when compared to other carbon based materials. Moreover, they have unique properties such as high surface area, modulated pore size, excellent thermal/electrical conductivity, and various oxygen surface functional groups.6–9 Furthermore, there are only a very few reports on the production of ACs from pumpkin stem precursors by using phosphoric acid for the application of phenol and chlorophenol adsorption.10,11 Nevertheless, to the best of our knowledge by an extensive literature survey, pumpkin stem waste derived activated carbon has not been explored for any solar cell applications.
Herein, we report a synthesis of novel functional micro–meso porous carbon with high surface area (∼793 m2 g−1) and modulated pore size. The as-synthesized AC samples were utilized as efficient CEs in DSSCs applications.
Scheme 1 demonstrates a schematic diagram for the preparation of pumpkin stem-derived activated carbon materials. Briefly, the pumpkin stem-derived activated carbon was synthesized by a simple chemical activation method, ZnCl2 was used as an activating agent.6,12 The sun-dried pumpkin stems (Cucurbita pepo) were washed thoroughly, pulverized and dried in an oven at 100 °C. The dried stems powder was preheated to 150 °C for 48 h. Then, desired amounts of preheated sample were activated with 10% of ZnCl2, individually. Accordingly, they were carbonized at three different temperatures of 700, 800 and 900 °C for 2 h in a N2 atmosphere at a heating rate of 10 °C min−1, separately. The carbonized AC samples were washed with distilled water and 1 M HCl to remove the impurities, and were referred to as pure AC. Finally, the carbonized AC samples were dried at 100 °C overnight and ground well to get a fine powder.
Titanium dioxide (Degussa P-25, Eversolar), cis-bis(isothiocyanato) bis(2,2′-bipyridyl-4,4′-dicarboxylato) ruthenium(II) bis-tetrabutylammonium (D719 dye, Eversolar), ethylene glycol (EG, Merck), acetic acid (C2H4O2, Merck), titanium isopropxide TIPO (Ti{OCH(CH3)2}4, Alfa aesar), potassium iodide (KI, Merck), iodine (I, Merck) and absolute ethanol (Merck) were purchased and used as received without further purification.
TiO2 paste was prepared using titanium sol in the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, TIPO
10, TIPO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetic acid
acetic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethylene glycol. The sol was prepared by heating ethylene glycol to 50 °C. While stirring, TIPO was added drop by drop into the EG solution. Later, an appropriate amount of acetic acid was added and the temperature was increased to 100 °C. The solution was stirred at this temperature until it turned clear. Then the TiO2 paste was prepared by grinding TiO2 powder and sol in a mortar for 1 h. The prepared paste was coated on the ITO coated glass plate by the doctor blade technique and sintered at 400 °C for 30 min. The photoanode was prepared by immersing the slightly warmed TiO2 film into 0.5 mM of Ru dye solution for 12 h. The non-adsorbed dye was washed off with acetonitrile. In the AC-CE preparation, 100 mg of the as-synthesized AC was ground with a corresponding amount of mineral oil for 30 min and coated on the ITO glass plate using the doctor blade technique and sintered at 400 °C for 1 h. The AC-CEs and dye coated TiO2 films were placed facing each other and held together using binder clips. The electrolyte solution was prepared by mixing 0.127 g of iodine and 0.83 g of potassium iodide (KI) in 10 ml of ethylene glycol. The iodide electrolyte solution was injected between the anode and cathode.
ethylene glycol. The sol was prepared by heating ethylene glycol to 50 °C. While stirring, TIPO was added drop by drop into the EG solution. Later, an appropriate amount of acetic acid was added and the temperature was increased to 100 °C. The solution was stirred at this temperature until it turned clear. Then the TiO2 paste was prepared by grinding TiO2 powder and sol in a mortar for 1 h. The prepared paste was coated on the ITO coated glass plate by the doctor blade technique and sintered at 400 °C for 30 min. The photoanode was prepared by immersing the slightly warmed TiO2 film into 0.5 mM of Ru dye solution for 12 h. The non-adsorbed dye was washed off with acetonitrile. In the AC-CE preparation, 100 mg of the as-synthesized AC was ground with a corresponding amount of mineral oil for 30 min and coated on the ITO glass plate using the doctor blade technique and sintered at 400 °C for 1 h. The AC-CEs and dye coated TiO2 films were placed facing each other and held together using binder clips. The electrolyte solution was prepared by mixing 0.127 g of iodine and 0.83 g of potassium iodide (KI) in 10 ml of ethylene glycol. The iodide electrolyte solution was injected between the anode and cathode.
The N2 adsorption–desorption isotherms and the pore size distribution were studied using a “Micromeritics ASAP 2020”. An Oriel class-A solar simulator (91195A, Newport) was used as a light source and a computer-controlled Autolab PGSTAT302N electrochemical workstation was employed for current–voltage (I–V) measurements. The elemental analysis was performed using an “elementar Vario EL cube” (for CHNS, German). The surface morphology of the film was studied using JEOL field-emission scanning electron microscopy. Raman scattering measurements were performed in 180° backscattering geometry using a LabRam HR800 spectrometer from Horiba Jobin Yvon equipped with a CCD detector. The sample was excited by 633 nm emission from a He–Ne laser and the accuracy of the wave number was about 0.3 cm−1.
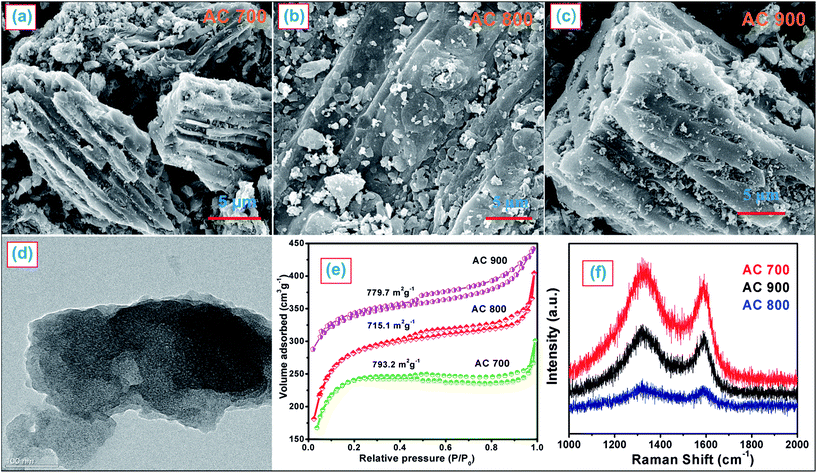
Fig. 1 shows the typical FE-SEM and HR-TEM images, N2 adsorption and desorption isotherms, and Raman spectra of the ACs. Fig. 1a–c shows the FE-SEM images of ACs at different temperatures, designated as AC700, AC800 and AC900 °C, which show the highly porous nature of the ACs. A further HR-TEM (Fig. 1d) image shows that AC700 exhibits a porous morphology. The highly porous nature of the ACs means they may act as reservoirs which allow access to the ions from the electrolyte, leading to enhanced catalytic activity. Fig. 1e shows the N2 sorption studies of the ACs. The ACs have surface areas of 793.2, 715.1, and 779.7 m2 g−1 for AC700, AC800, and AC900, respectively, as calculated by the Brunauer–Emmett–Teller (BET) model. The pore volume (Fig. S1†) of the ACs has been calculated as 0.4, 0.35 and 0.36 cm3 g−1, respectively, using the BJH model, and the micro–mesopore and mesopore distributions of AC samples are in the range of ∼1–3 nm.
Raman spectroscopy is a sensitive method which provides a wealth of information on the structure of carbonaceous materials. The Raman studies of the ACs are shown in Fig. 1f. The Raman spectra of the ACs show a D band (∼1320 cm−1, ring breathing mode from sp2 carbon rings, A1g mode) and a G band (1592 cm−1, planar configuration sp2 bonded carbon with bond-stretching motion, E2g mode). The ratio of the intensities of the D and G bands is 0.91 for the AC.10 Moreover, the intensity of the D band is higher than the G band, which indicates the AC is amorphous in nature. In addition, Table 1 shows the CHNS elemental analysis, which confirms the presence of heteroatoms like carbon (83.427%), hydrogen (0.861%), nitrogen (1.085%), and sulfur (0.383%) in the AC.7,8
| AC700 weight (mg) | C atom/% | N atom/% | S atom/% | H atom/% |
|---|---|---|---|---|
| a C – carbon, N – nitrogen, S – sulfur, H – hydrogen. | ||||
| 2.206 | 83.465 | 1.074 | 0.386 | 0.873 |
| 2.114 | 83.389 | 1.096 | 0.380 | 0.849 |
| Mean | 83.427 | 1.085 | 0.383 | 0.861 |
In order to analyze the specific surface characteristics of the AC electrodes, they were characterized by cyclic voltammetry (CV) in a three-electrode system in 1.0 M LiClO4 as the electrolyte. As shown in Fig. 2A, the AC700 electrode exhibits a higher capacitive current density than the other AC electrodes, which is consistent with the BET surface area analysis, with the higher surface area and more active sites leading to more efficient charge accumulation. Hence, the higher surface area of the AC700 plays an important role to enhance the current density through the fast accumulation of electrical charges at the electrode/electrolyte interfaces.13
Furthermore, CV was performed to understand the ion diffusivity and electrocatalytic activity of the AC electrodes containing 10 mM KI, 1.0 mM I2, and 0.1 M LiClO4 in acetonitrile in the same three electrode system. As shown in Fig. 2B, for all AC electrodes we observed two pairs of redox peaks, the anodic peak shows the oxidation of iodide and tri-iodide (eqn (1)), and the cathodic peak shows the reduction of tri-iodide (eqn (2)).14
| 3I2 + 2e− = 2I3− | (1) |
| I3− + 2e− = 3I− | (2) |
Among the AC electrodes, AC700 possesses a higher cathodic peak current density (2 mA cm−2) than AC800 (1.71 mA cm−2) and AC900 (1.85 mA cm−2). This result also confirms that the AC700 has higher electrocatalytic activity, and is an efficient counter electrode for the I3− reaction (eqn (3)).15
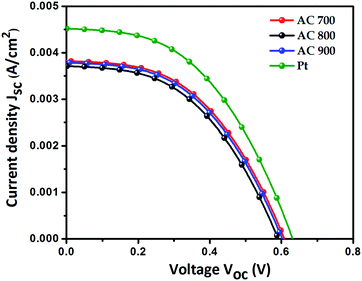
The J–V performance of devices based on the ACs is shown in Fig. 3. For J–V measurement, the devices were subjected to simulated sunlight irradiation with a power output of about 40 mW cm−2. The effective area of the working electrode was 1 cm2 (1 cm × 1 cm). The detailed photovoltaic parameters, such as the open-circuit voltage (Voc), fill factor (FF), short-circuit current density (Jsc), and power conversion efficiency (PCE) are summarized in Table 2.
| S. no. | Sample name | Surface area (m2 g−1) | Jsc (A) | Voc (V) | FF | η (%) |
|---|---|---|---|---|---|---|
| 1. | AC700 | 793 | 0.00384 | 0.611 | 0.475 | 2.79 |
| 2. | AC800 | 715 | 0.00370 | 0.589 | 0.464 | 2.53 |
| 3. | AC900 | 779 | 0.00378 | 0.603 | 0.487 | 2.77 |
| 4. | Pt | — | 0.00454 | 0.736 | 0.493 | 4.04 |
The fill factor and the efficiency of the cells were calculated by the following equations.
| FF = (VmJm/VocJsc) × 100 | (3) |
| η = (VocJscFF/Pin) × 100 | (4) |
As shown in Fig. 3, it can be seen that the AC counter electrodes have good photovoltaic performance. As shown, the DSSCs fabricated with the AC700, AC800 and AC900 and Pt counter electrodes showed overall conversion efficiencies of 2.79, 2.53, 2.77, and 4.04%, respectively. The obtained efficiencies are comparable with many previously reported graphene electrodes (see Table S1†). The comparable photoelectrochemical performance of the AC-CEs with Pt mainly comes from the highly porous nature and surface area of the AC-CEs, which leads to facile charge transfer through promoting the I3−/I− redox reaction rate by easily taking up the liquid electrolyte into their micro–mesopores. Thus, the increase in contact area between the AC and liquid electrolyte caused the excellent redox reaction rate. The AC700 has larger specific surface area (793.2 m2 g−1) than AC800 and AC900 (715 m2 g−1, 779 m2 g−1), and thus gives higher conversion efficiency.1,12–14
In conclusion, ACs with a high surface area are prepared by a simple and eco-friendly method. The AC material exhibited a significant performance toward DSSCs applications. The DSSCs made with the AC700 counter electrode exhibited a higher PCE of 2.79%. The overall performance is more comparable with reported graphene based counter electrodes. The enhanced performance may be attributed to the high surface area, micro–mesoporous behavior and the presence of oxygen surface functional groups of the activated carbon.
Acknowledgements
This project was supported by the National Science Council and the Ministry of Education of Taiwan (Republic of China).Notes and references
- M. Gratzel, J. Photochem. Photobiol., C, 2003, 4, 145–153 CrossRef CAS.
- M. Gratzel, Inorg. Chem., 2005, 44, 6841–6851 CrossRef PubMed.
- T. Battumur, S. H. Mujawar, Q. T. Truong, S. B. Ambade, D. S. Lee, W. Lee, S. H. Han and S. H. Lee, Curr. Appl. Phys., 2012, 12, 49–53 CrossRef PubMed.
- N. Murakami and M. Gratzel, Inorg. Chim. Acta, 2008, 361, 572–580 CrossRef PubMed.
- H. Choi, H. Kim, S. Hwang, Y. Han and M. Jeon, J. Mater. Chem., 2011, 21, 7548–7551 RSC.
- M. Sevilla and R. Mokaya, Energy Environ. Sci., 2014, 7, 1250–1280 CAS.
- R. Madhu, V. Veeramani and S.-M. Chen, Sci. Rep., 2014, 4, 4679, DOI:10.1038/srep04679.
- R. Madhu, K. Vijaya Sankar, S.-M. Chen and R. K. Selvan, RSC Adv., 2014, 4, 1225–1233 RSC.
- Z. Huang, X. Liu, K. Li, D. Li, Y. Luo, H. Li, W. Songa and L. Q. Chen, Electrochem. Commun., 2007, 9, 596–598 CrossRef CAS PubMed.
- O. A. Ekpete, M. J. Horsfall and T. Tarawou, J. Appl. Sci. Environ. Manage., 2011, 15, 141–146 CAS.
- O. A. Ekpete and M. J. Horsfall, Res. J. Chem. Sci., 2011, 1, 10–17 CAS.
- Y. A. Alhamed, Eng. Sci., 2006, 17, 75–100 Search PubMed.
- M. H. Yeh, L. Y. Lin, C. L. Sun, Y. A. Leu, J. T. Tsai, C. Y. Yeh, R. Vittal and K. C. Ho, J. Phys. Chem. C, 2014, 118(30), 16626–16634 CAS.
- T. C. Wei, C. C. Wan, Y. Y. Wang, C. M. Chen and H. S. Shiu, J. Phys. Chem. C, 2007, 111, 4847–4853 CAS.
- Z. Huang, X. Liu, K. Li, D. Li, Y. Luo, H. Li, W. Songa, L. Chen and Q. Meng, Electrochem. Commun., 2007, 9, 596–598 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra12585a |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2014 |