Cascade reaction-based rapid and ratiometric detection of H2S/S2− in the presence of bio-thiols with live cell imaging: demasking of ESIPT approach†
Shyamaprosad Goswami*a,
Abhishek Mannaa,
Monalisa Mondala and
Debasish Sarkarb
aIndian Institute of Engineering Science and Technology (formerly Bengal Engineering and Science University), Shibpur, Howrah 711103, India. E-mail: spgoswamical@yahoo.com; Fax: +91-3326682916
bDepartment of Life Science and Biotechnology, Jadavpur University, Kolkata, India
First published on 5th November 2014
Abstract
For the rapid, ratiometric, fluorogenic and “naked eye” detection of H2S/S2−, a pro-excited state intramolecular proton transfer (ESIPT)-based receptor, 2-formyl-benzoic acid 2-benzothiazol-2-yl-phenyl ester (FBBP) has been designed. In the presence of H2S/S2− in preference to other common species, 2-(2-hydroxyphenyl)benzothiazole, a well known ESIPT-containing agent, is recovered from FBBP. Thus, H2S/S2− can be detected in vitro and in vivo by such a simple, easy-to-synthesise, practical ratiometric sensor.
Introduction
After nitrogen oxide and carbon monoxide, hydrogen sulfide (H2S) has recently become known as the third of the principal gasotransmitters for regulating cardiovascular, neuronal, immune, endocrine and gastrointestinal systems.1 H2S is created endogenously from sulfur-containing molecules by the enzymes cystathionine β-synthase, cystathionine γ-lyase, and 3-mercaptopyruvate sulfurtransferase in the cytosols and mitochondria of mammalian cells.2 Endogenous levels of H2S play an important role in a vast number of physiological and pathological processes, such as regulation of vascular function and protection of cells from oxidative stress and vascular injury.3 Thus, abnormal levels of H2S are related to a series of diseases, such as diabetes, hypertension, stroke, Alzheimer's, Down's syndrome and cirrhosis of the liver.4Therefore, visualization of the distribution and concentration of H2S in living systems would be very significant and helpful in clarifying the biological roles of H2S. Compared with reported methods such as colorimetric,5 electrochemical analysis,6 and gas chromatography,7 small molecule fluorescent probes present high sensitivity, real-time imaging, and high spatiotemporal resolution and have excellent potential to be useful tools.
Alternatively, ratiometric fluorescent chemosensors are of particular interest because of their simplicity.8 In particular, ratiometric sensing provides a way of avoiding any misinterpretation of analyte-induced fluorescence quenching or enhancement because of photobleaching, sensor concentration, and medium effects.9 A ratiometric method measuring the ratio of fluorescence intensities at two wavelengths provides an alternative approach. However, up to now, only a limited number of ratiometric fluorescence probes for H2S have been reported in the literature.10
Based on the H2S-mediated reduction of azide (or nitro or hydroxyamine) to amine,11 some groups have recently made great progress in the recognition of H2S in vitro and in vivo. An H2S-mediated nucleophilic addition reaction,12 or a displacement strategy based on copper sulfide precipitation,13 has also proved to be a convenient approach for this purpose. Yet, one of the factors limiting the extensive use of many probes is the multi-step synthesis required to obtain the receptor. Furthermore, most of the free fluorescent probes for H2S have relatively low intensity with a “turn on” approach, which is a great barrier for real-time applications. Therefore, it is still necessary to devise simple fluorescent H2S probes with a large rapid ratiometric response, which can be used for H2S detection under physiological conditions.
However, because of their intrinsic properties, notably an ultra-fast reaction rate and an exceptionally large fluorescence Stokes shift,14 great interest has been shown in excited state intramolecular proton transfer (ESIPT) compounds. Upon irradiation, 2-(2-hydroxyphenyl)benzothiazole (HBT) and its derivatives generate the ESIPT tautomers (the keto forms), which show fluorescence more strongly at longer wavelengths compared to the phenol forms. In recent times, researchers have also devised anion and cation sensors using the ESIPT method, with HBT as an ideal moiety for this purpose.15 We also chose the well-characterised HBT as an ESIPT-containing moiety to construct a real-time probe for H2S detection.
Thus, in this paper, a new cascade reaction (nucleophilic addition, cyclisation and elimination)-based fluorogenic reactive probe, FBBP, is reported, which can be used to detect H2S with a rapid response.
Experimental results and explanation
General
The chemicals and solvents were purchased from Sigma-Aldrich Chemicals and were used without further purification. Melting points were determined using a hot-plate melting point apparatus with an open-mouth capillary and were uncorrected. Proton nuclear magnetic resonance (1H-NMR) and 13C-NMR spectra were recorded on 500 MHz and 100 MHz instruments, respectively. For NMR spectra, deuterated chloroform (CDCl3) was used as solvent with trimethyl siloxane as an internal standard. Chemical shifts are expressed in δ units and 1H–1H coupling constants in Hz. Fluorescence experiments were performed using a PTI fluorescence spectrophotometer, and using a fluorescence cell with a 10 mm path. The live cell imaging was carried out using a Leica DM2500 fluorescence microscope.Experimental procedure
Fluorescence study
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O) (2
H2O) (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, v/v) at pH 7.4 using 10 mM HEPES buffer. The solution of the guest anion was prepared (2 × 10−5 M l−1) in H2O at pH 7.4 using 10 mM HEPES buffer. The original volume of the receptor solution was 2 ml. Solutions of the sensor at various concentrations and of increasing concentrations of cations, anions and amine-containing compounds were prepared separately. The spectra of these solutions were recorded using fluorescence methods.
8, v/v) at pH 7.4 using 10 mM HEPES buffer. The solution of the guest anion was prepared (2 × 10−5 M l−1) in H2O at pH 7.4 using 10 mM HEPES buffer. The original volume of the receptor solution was 2 ml. Solutions of the sensor at various concentrations and of increasing concentrations of cations, anions and amine-containing compounds were prepared separately. The spectra of these solutions were recorded using fluorescence methods.Results and discussion
The synthesis of the sensor is shown in Scheme 1 (details of the procedure and spectra are given in the electronic ESI†).It is well known that HBT and its derivatives produce the ESIPT tautomers (the keto forms), which show fluorescence more powerfully at longer wavelengths compared to the phenol forms upon irradiation. The enol isomer, which is lower in energy than the keto isomer in the electronic ground state, undergoes the proton transfer reaction upon excitation to the excited state. As shown in Fig. 1, FBBP itself exhibits emission at 359 nm (excitation at 310 nm). With the addition of only 0.5 μM of S2−, the emission at 359 nm decreased, followed by a new peak appearing at 462 nm. This indicated that the chemical reaction between sulfide ions and the receptor (FBBP) started at this minimum concentration and thus the ESIPT properties of HBT were demasked (Scheme 2).
Accordingly, a color change from colorless to blue, as well as an emission with a well-defined iso-emissive point at 417 nm, was observed. Essentially, these changes in the fluorescence spectrum stopped and the ratio of the emission intensities at 359 nm and 462 nm (I462/I359) became constant when the amount of S2− added reached 1.2 equiv. It is noteworthy that the difference in the two emission wavelengths is very large (emission shift: ΔF = 103 nm), which not only contributes to the accurate measurement of the intensities of the two emission peaks, but also results in a huge ratiometric value. In fact, in the presence of 1.2 equiv. of S2−, an approximate 30-fold enhancement in the ratiometric value (I462/I359 from 0.04 to 1.45) is achieved compared to that obtained with the sulfide-free solution (Fig. 1).
To evaluate the selective nature of H2S/S2− towards FBBP, spectral emission changes upon addition of 5 equivalents of common interfering species, i.e., cysteine (Cys), glutathione (GSH), hydrogen peroxide (H2O2), fluoride (F−), cyanide (CN−), azide (N3−), hypochlorite (OCl−), hydroxyl radical (OH˙), thiocyanide (SCN−), sulfite (SO32−), hydrogen sulfite (HSO3−), thiosulfate (S2O32−) and ascorbic acid, were studied. As shown in Fig. 2, in most of these cases, no change in emission intensity ratio (I462/I359) was noted. The selectivity observed by emission monitoring matched, when FBBP was employed as a colorimetric sensor for the detection of sulfide ions. In contrast, a visual color change from colorless to bright blue was observed (under UV light) and was associated with the reaction of FBBP with sulfide ions.
 | ||
| Fig. 2 The bar plot of the ratiometric response of FBBP in the presence of all the tested anions (5 equiv., except for S2−, which was 1.2 equiv.). | ||
The selectivity of the FBBP probe was also checked by quantitatively recording the fluorescence intensity of FBBP in the presence of an excess of 10 times the concentration of different types of interfering species (Fig. S3, ESI†). Most of the other species exhibited no effect on the FBBP detection of S2−. Thus, these results demonstrated that FBBP has a sensitive response towards S2−.
No significant color change was promoted by the addition of other anions under UV light. From the titration data (Fig. 3), it is clear that a concentration of 1.5 μM S2− is enough for the ratiometric response using this unique probe. Under these conditions, the changes in the intensity of the two peaks (i.e., I462 and I359) produced an excellent linear function with respect to the concentration of sulfide between 3 and 12 μM (Fig. 4).
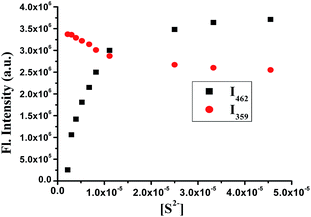 | ||
| Fig. 3 Plot of [S2−] versus fluorescence intensity of FBBP at two different wavelengths, i.e., at 462 and 359 nm (λex = 310 nm). | ||
 | ||
| Fig. 4 Emission intensity ratio of FBBP versus [S2−] between 3 to 12 μM of [S2−] and the overall change of intensity ratio versus [S2−] (inset). | ||
The detection limit for S2− was determined to be 0.51 μM using the probe, i.e., FBBP based on K × Sb1/S, where Sb1 is the standard deviation and S is the slope of the calibration curve (see ESI†).
Using time-dependent fluorescence spectra, it was shown that the reaction was completed within approximately 3 min, with a rate constant of 1.9 × 10−2 s−1, which strongly supports the high reactivity of the probe (Fig. S2, ESI†).
Predicted chemical mechanism for H2S detection
It was envisaged that the observed change in presence of sulfide may arise from the HBT moiety which is released from FBBP with a cascade type of reaction between S2− and the reactive probe, i.e., FBBP. The formation of HBT from FBBP was confirmed by its 1H-NMR structure. The characteristic aldehyde proton of FBBP disappeared with the appearance of a new phenolic proton peak of HBT when 1 equivalent of Na2S solution was added to the solution of FBBP. The mass spectrum (ESI-MS) of the product after mixing with Na2S shows a peak at m/z 227.04, which is possibly HBT, and at m/z 166.0, which is possibly a side product, which also proves a cascade-based reaction of S2− towards FBBP, m/z 359.06 (see ESI†).Detection of H2S in living cells
It was then decided to examine whether FBBP could sense H2S in living cells. Yeast (Saccharomyces cerevisiae) cells were grown in 5 ml of YPD broth (1% yeast extract, 2% peptone and 2% dextrose) overnight at 30 °C. Next day, a fresh 30 ml of YPD broth was inoculated with a sample from the overnight culture and grown till mid log phase (0.6–1.0 OD at 600 nm). FBBP (50 μM) was added to the culture and cells were grown for up to three hours. A portion (1 ml) of the cell culture was harvested and washed with phosphate-buffered saline. Finally the cell pellet was dissolved in 500 μl of ultrapure water (Sartorius Milli-Q) supplemented with 50 μM of Na2S. Then imaging was carried out using a Leica DM2500 fluorescence microscope.It can be seen that, after 30 min incubation with Na2S solution, there was a higher fluorescence turn-on (Fig. 5). These experiments indicate that FBBP can be used to detect H2S in living cells. The MTT assay for the probe, i.e., FBBP, was conducted, and the results showed that the receptor could be used safely at a concentration of 50 μM (Fig. S24, ESI†).
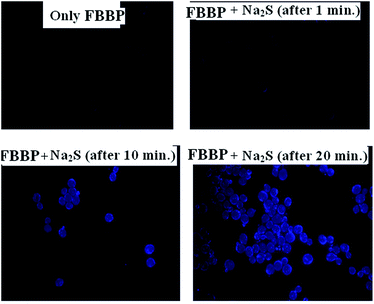 | ||
| Fig. 5 Fluoresence image of a yeast cell treated with FBBP alone, and with FBBP with the addition of Na2S (at time intervals of 1 min, 10 min and 20 min). | ||
Conclusion
In conclusion, a simple receptor (FBBP) has been designed and synthesized for the selective, sensitive and ratiometric rapid response towards sulfide with a chemodosimetric approach in aqueous media. From the spectral data, it is clear that the sensor can be used practically to detect H2S/S2− quantitatively with a high selectivity over other anions. The ESIPT-based sensing phenomenon is also useful for live cell imaging of H2S/S2−.Acknowledgements
We thank the DST and CSIR (Government of India) for financial support. Abhishek Manna acknowledges CSIR for providing a fellowship.Notes and references
- K. R. Olson and J. A. Donald, Acta Histochem., 2009, 111, 244 CrossRef CAS PubMed; R. Schulz, M. Kelm and G. Heusch, Cardiovasc. Res., 2004, 61, 402 CrossRef; X. Liu, G. Chapman, K. Peyton, A. Schafer and W. Durante, Cardiovasc. Res., 2002, 55, 396 CrossRef; R. Wang, Antioxid. Redox Signaling, 2003, 5, 493 CrossRef.
- L. Li, P. Rose and P. K. Moore, Annu. Rev. Pharmacol. Toxicol., 2011, 51, 169 CrossRef CAS.
- D. R. Linden, J. Furne, G. J. Stoltz, M. S. Abdel-Rehim, M. D. Levitt and J. H. Szurszewski, Br. J. Pharmacol., 2012, 165, 2178 CrossRef CAS; R. G. Hendrickson, A. Chang and R. J. Hamilton, Am. J. Ind. Med., 2004, 45, 346 CrossRef; R. J. Reiffenstein, W. C. Hulbert and S. H. Roth, Annu. Rev. Pharmacol. Toxicol., 1992, 32, 109 CrossRef; C. E. Paulsen and K. S. Carroll, Chem. Rev., 2013, 113, 4633 CrossRef; K. Kashfi and K. R. Olson, Biochem. Pharmacol., 2013, 85, 689 CrossRef PubMed; M. Fu, W. Zhang, L. Wu, G. Yang, H. Li and R. Wang, Proc. Natl. Acad. Sci., 2012, 109, 2943 CrossRef PubMed; L. Li, P. Rose and P. K. Moore, Annu. Rev. Pharmacol. Toxicol., 2011, 51, 169 CrossRef PubMed; K. Shatalin, E. Shatalina, A. Mironov and E. Nudler, Science, 2011, 334, 986 CrossRef PubMed; O. Kabil and R. Banerjee, J. Biol. Chem., 2010, 285, 21903 CrossRef PubMed.
- M. E. Dodge and L. Lum, Annu. Rev. Pharmacol. Toxicol., 2011, 51, 289 CrossRef CAS PubMed; C. Szabó, Nat. Rev. Drug Discovery, 2007, 6, 917 CrossRef PubMed; P. Kamoun, M.-C. Belardinelli, A. Chabli, K. Lallouchi and B. Chadefaux-Vekemans, Am. J. Med. Genet., Part A, 2003, 116, 310 CrossRef PubMed; S. Fiorucci, E. Antonelli, A. Mencarelli, S. Orlandi, B. Renga, G. Rizzo, E. Distrutti, V. Shah and A. Morelli, Hepatology, 2005, 42, 539 CrossRef PubMed; R. J. Reiffenstein, W. C. Hulbert and S. H. Roth, Annu. Rev. Pharmacol. Toxicol., 1992, 32, 109 CrossRef; L. Li and P. K. Moore, Trends Pharmacol. Sci., 2008, 2, 84 CrossRef.
- M. G. Choi, S. Cha, H. Lee, H. L. Jeon and S. K. Chang, Chem. Commun., 2009, 7390 RSC.
- N. S. Lawrence, J. Davis, L. Jiang, T. G. J. Jones, S. N. Davies and R. G. Compton, Electroanalysis, 2000, 18, 1453 CrossRef.
- J. Furne, A. Saeed and M. D. Levitt, Am. J. Physiol., 2008, 295, R1479 CAS.
- P. D. Beer and P. A. Gale, Angew. Chem., Int. Ed., 2001, 40, 486 CrossRef CAS; V. Amendola, D. Esteban-Gómez, L. Fabbrizzi and M. Licchelli, Acc. Chem. Res., 2006, 39, 343 CrossRef; R. Martinez-Manez and F. Sancenon, Chem. Rev., 2003, 103, 4419 CrossRef; S. J. Brooks, P. A. Gale and M. E. Light, Chem. Commun., 2005, 4696 RSC; S. J. Brooks, C.-Y. Wu, M.-S. Chen, C.-A. Lin, S.-C. Lin and S.-S. Sun, Chem.–Eur. J., 2006, 12, 2263 CrossRef.
- A. Ajayaghosh, P. Carol and S. Sreejith, J. Am. Chem. Soc., 2005, 127, 14962 CrossRef CAS.
- L. Zhang, S. Li, M. Hong, Y. Xu, S. Wang, Y. Liu, Y. Qian and J. Zhao, Org. Biomol. Chem., 2014, 12, 5115 Search PubMed; M.-Y. Wu, K. Li, J.-T. Hou, Z. Huang and X.-Q. Yu, Org. Biomol. Chem., 2012, 10, 8342 Search PubMed; B. Wang, P. Li, F. Yu, J. Chen, Z. Qu and K. Han, Chem. Commun., 2013, 49, 5790 RSC; Y. Yang, C. Yin, F. Huo, Y. Zhang and J. Chao, Sens. Actuators, B, 2014, 203, 596 CrossRef CAS PubMed; Q. Huang, X.-F. Yang and H. Li, Dyes Pigm., 2013, 99, 871 CrossRef PubMed; N. Kumar, V. Bhalla and M. Kumar, Coord. Chem. Rev., 2013, 257, 2335 CrossRef PubMed.
- A. R. Lippert, E. J. New and C. J. Chang, J. Am. Chem. Soc., 2011, 133, 10078 CrossRef CAS PubMed; H. Zhang, P. Wang, G. Chen, H.-Y. Cheung and H. Sun, Tetrahedron Lett., 2013, 54, 4826 CrossRef PubMed; L. A. Montoya and M. D. Pluth, Chem. Commun., 2012, 48, 4767 RSC; B. Chen, C. Lv and X. Tang, Anal. Bioanal. Chem., 2012, 404, 1919 CrossRef PubMed; G. Zhou, H. Wang, Y. Ma and X. Chen, Tetrahedron, 2013, 69, 867 CrossRef PubMed; S. K. Das, C. S. Lim, S. Y. Yang, J. H. Han and B. R. Cho, Chem. Commun., 2012, 48, 8395 RSC; Z. Wu, Z. Li, L. Yang, J. Han and S. Han, Chem. Commun., 2012, 48, 10120 RSC; B. Chen, W. Li, C. Lv, M. Zhao, H. Jin, H. Jin, J. Du, L. Zhang and X. Tang, Analyst, 2013, 138, 946 RSC; Q. Wan, Y. Song, Z. Li, X. Gao and H. Ma, Chem. Commun., 2013, 49, 502 RSC; H. Peng, Y. Cheng, C. Dai, A. L. King, B. L. Predmore, D. J. Lefer and B. Wang, Angew. Chem., Int. Ed., 2011, 50, 9672 CrossRef PubMed; J. Zhou, Y. Luo, Q. Li, J. Shen, R. Wang, Y. xu and X. H. Qian, New J. Chem., 2014, 38, 2770 RSC; K. Zheng, W. Lin and L. Tan, Org. Biomol. Chem., 2012, 10, 9683 Search PubMed; W. Xuan, R. Pan, Y. Cao, K. Liu and W. Wang, Chem. Commun., 2012, 48, 10669 RSC; V. S. Lin, A. R. Lippert and C. J. Chang, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 7131 CrossRef PubMed; W. Sun, J. Fan, C. Hu, J. Cao, H. Zhang, X. Xiong, J. Wang, S. Cui, S. Sun and X. Peng, Chem. Commun., 2013, 49, 3890 RSC; J. Bae, M. G. Choi, J. Choi and S.-K. Chang, Dyes Pigm., 2013, 99, 748 CrossRef PubMed; Y. Cai, L. Li, Z. Wang, J. Z. Sun, A. Qin and B. Z. Tang, Chem. Commun., 2014, 50, 8892 RSC; Y. Jiang, Q. We and X. Chang, Talanta, 2014, 121, 122 CrossRef PubMed.
- C. Liu, J. Pan, S. Li, Y. Zhao, L. Y. Wu, C. E. Berkman, A. R. Whorton and M. Xian, Angew. Chem., Int. Ed., 2011, 50, 10327 CrossRef CAS PubMed; Y. Qian, J. Karpus, O. Kabil, S.-Y. Zhang, H.-L. Zhu, R. Banerjee, J. Zhao and C. He, Nat. Commun., 2011, 2, 495 CrossRef; X. Chen, S. Wu, J. Han and S. Han, Bioorg. Med. Chem. Lett., 2013, 23, 5295 CrossRef PubMed; X. Li, S. Zhang, J. Cao, N. Xie, T. Liu, B. Yang, Q. He and Y. Hu, Chem. Commun., 2013, 49, 8656 RSC; Q. Huang, X.-F. Yang and H. Li, Dyes Pigm., 2013, 99, 871 CrossRef; Y. Chen, C. Zhu, Z. Yang, J. Chen, Y. He, Y. Jiao, W. He, L. Qiu, J. Cen and Z. Guo, Angew. Chem., Int. Ed., 2013, 52, 1688 CrossRef; D. Maity, A. Raj, P. K. Samanta, D. Karthigeyan, T. K. Kundu, S. K. Pati and T. Govindaraju, RSC Adv., 2014, 4, 11147 RSC; X. Wang, J. Sun, W. Zhang, X. Ma, J. Lv and B. Tang, Chem. Sci., 2013, 4, 2551 RSC; C. Liu, H. Wu, B. Han, B. Zhu and X. Zhang, Dyes Pigm., 2014, 110, 214 CrossRef PubMed; Y. Liu and G. Feng, Org. Biomol. Chem., 2014, 12, 438 Search PubMed; J. Zhang, Y.-Q. Sun, J. Liu, Y. Shi and W. Guo, Chem. Commun., 2013, 49, 11305 RSC; Y. Qian, L. Zhang, S. Ding, X. Deng, C. He, X. E. Zheng, H.-L. Zhu and J. Zhao, Chem. Sci., 2012, 3, 2920 RSC; Y. Chen, C. Zhu, Z. Yang, J. Chen, Y. He, Y. Jiao, W. He, L. Qiu, J. Cen and Z. Guo, Angew. Chem., Int. Ed., 2013, 52, 1688 CrossRef PubMed; J. Liu, Y.-Q. Sun, J. Zhang, T. Yang, J. Cao, L. Zhang and W. Guo, Chem.–Eur. J., 2013, 19, 4717 CrossRef PubMed; T. Liu, Z. Xu, D. R. Spring and J. Cui, Org. Lett., 2013, 15, 2310 CrossRef PubMed.
- K. Sasakura, K. Hanaoka, N. Shibuya, Y. Mikami, Y. Kimura, T. Komatsu, T. Ueno, T. Terai, H. Kimura and T. Nagano, J. Am. Chem. Soc., 2011, 133, 18003 CrossRef CAS PubMed; F. Hou, L. Huang, P. Xi, J. Cheng, X. Zhao, G. Xie, Y. Shi, F. Cheng, X. Yao, D. Bai and Z. Zeng, Inorg. Chem., 2012, 51, 2454 CrossRef; X. Wu, H. Li, Y. Kan and B. Yin, Dalton Trans., 2013, 42, 16302 RSC; J.-T. Hou, B.-Y. Liu, K. Li, K.-K. Yu, M.-B. Wu and X.-Q. Yu, Talanta, 2013, 116, 434 CrossRef PubMed; X. Qu, C. Li, H. Chen, J. Mack, Z. Guo and Z. Shen, Chem. Commun., 2013, 49, 7510 RSC.
- S. Kim, J. Seo, H. K. Jung, J. J. Kim and S. Y. Park, Adv. Mater., 2005, 17, 2077 CrossRef CAS; T. Mutai, H. Tomoda, T. Ohkawa, Y. Yabe and K. Araki, Angew. Chem., Int. Ed., 2008, 47, 9522 CrossRef PubMed.
- I. T. Kim, J. H. Kang, G. Han, J. S. Chung and Y. Kim, Chem. Commun., 2009, 45, 5895 RSC; R. Hu, J. Feng, D. Hu, S. Wang, S. Li, Y. Li and G. Yang, Angew. Chem., Int. Ed., 2010, 49, 4915 CrossRef CAS PubMed; H. W. Chen, Y. Xing and Y. Pang, Org. Lett., 2011, 13, 1362 CrossRef PubMed; L. Wang, Q. Zang, W. Chen, Y. Hao, Y.-N. Liu and J. Li, RSC Adv., 2013, 3, 8674 RSC; S. Liu, L. Zhang, W. Zan, X. Yao, Y. Yang and X. Liu, Sens. Actuators, B, 2014, 192, 386 CrossRef PubMed; L. Tang, M. Cai, P. Zhou, J. Zhao, K. Zhong, S. Hou and Y. Bian, RSC Adv., 2013, 3, 16802 RSC; B. Liu, H. Wang, T. Wang, Y. Bao, F. Du, J. Tian, Q. Li and R. Bai, Chem. Commun., 2012, 48, 2867 RSC; B. Liu, H. Wang, T. Wang, Y. Bao, F. Du, J. Tian, Q. Li and R. Bai, Chem. Commun., 2012, 48, 2867 RSC; V. Luxami and S. Kumar, RSC Adv., 2012, 2, 8734 RSC; Z. Xu, L. Xu, J. Zhou, Y. Xu, W. Zhu and X. Qian, Chem. Commun., 2012, 48, 10871 RSC; S. Goswami, A. Manna, S. Paul, A. K. Das, K. Aich and P. K. Nandi, Chem. Commun., 2013, 49, 2912 RSC; S. Goswami, A. Manna, S. Paul, A. K. Das, A. K. Maity, P. Saha and P. K. Nandi, Tetrahedron Lett., 2014, 55, 490 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Details of synthetic procedure and spectral data available. See DOI: 10.1039/c4ra12537a |
| This journal is © The Royal Society of Chemistry 2014 |



