Signal on fluorescence biosensor for MMP-2 based on FRET between semiconducting polymer dots and a metal organic framework
Weiqiang Yanga,
Guiyun Zhangac,
Wen Weng*b,
Bin Qiua,
Longhua Guoa,
Zhenyu Lin*a and
Guonan Chena
aMOE Key Laboratory of Analysis and Detection for Food Safety, Fujian Provincial Key Laboratory of Analysis and Detection Technology for Food Safety, Department of Chemistry, Fuzhou University, Fuzhou, 350002, China. E-mail: zylin @fzu.edu.cn
bDepartment of Chemistry and Environmental Science, Minnan Normal University, Zhangzhou, 363000, China. E-mail: weng_wen@126.com
cFood and Biological Engineering, Zhangzhou Institute of Technology, 363000, China
First published on 30th October 2014
Abstract
A signal on fluorescence biosensor for ultrasensitive detection of MMP-2 based on fluorescence resonance energy transfer (FRET) between semiconducting polymer dots (Pdots) and a metal–organic framework (MOF) has been developed. Carboxyl-rich PFO has been modified firmly with a polypeptide chain (COOH–GHHYYGPLGVRGC–NH2) through a carboxy ammonia reaction first. The polypeptide chain comprises the specific MMP-2 substrate domain (PLGVR) and the π-rich motif (HHYY) can be absorbed by the MOF (H2dtoaCu used in this study) through π-staking interactions. Hence, the fluorescence from Pdots can be quenched by the MOF with the assistance of the polypeptide chain linker through the FRET process. Upon the cleavage of the substrate by the protease (MMP-2) at the amide bond between Gly and Val, the Pdots has been separated from the MOF surface and therefore the FRET process can be inhibited, and the fluorescence of the system recovered. Under optimal conditions, the fluorescence recovery was found to be proportional to the concentration of MMP-2 within the range of 0.1 to 2.5 pg mL−1.
1. Introduction
Recently, semiconducting polymer dots (Pdots) have received increasing interest. Compared with traditional organic dyes or inorganic semiconductor quantum dots, semiconducting polymer dots (Pdots), made from conjugated polymer monomers, have characteristics of high fluorescence brightness, excellent photostability, nontoxic characteristics and easy surface modification.1–3 These materials had found their application in tumor targeting, and specific cell imaging frequently.4 They have been adopted as energy-acceptors in fluorescence resonance energy transfer (FRET) systems to engender or amplify signals.5,6 In our early study, a fluorescence biosensor for carcinoembryonic antigen (CEA) with high sensitivity and selectivity employing Pdots as luminophors had been developed,7 the results indicate Pdot is a promising new fluorescence probe. But till now, though which owns many attractive prospects, the research on the biosensor based on Pdots is still in its early stages.Metal–organic framework (MOF) is a novel class of porous, crystalline, 3D networked material consists of metal ions or clusters connected by organic linker groups.8 Which owns the characters of flexible porosity, high surface area and well-defined configuration,9 and had been applied frequently in the fields such as gas storage,10,11 heterogeneous catalysis,12 separations,13,14 and drug delivery.15 Many sensors for cations, anions, small molecules and vapors had been developed based on different features of the MOFs.16,17 Since the MOF consists π-electrons system, which could bind fluorophore-labelled ssDNAs and quench the fluorescence, based on which, many sensitive and selective biosensors for biomolecules and DNA sequences had been developed by using the traditional organic dyes as fluorophore.18,19 However, few studies were reported about the interaction between the Pdots and MOF, and the combined application of them for a practical detection was rather not investigated yet.
Matrix metalloproteinases (MMPs) are a family of zinc metalloproteinases with the function of degrading the extracellular matrix components and other extracellular proteins in processes of tissue remodeling.20,21 MMP-2, also known as gelatinase A,22 is a crucial member of MMPs, which is able to degrade type VI collagen and thus plays a key role in physiological and pathological states.23,24 Early studies showed that the concentration of MMP-2 in blood increase to vary degrees when different type of cancer, diabetes and hypertension occurs.25 Therefore, the quantification of MMP-2 is significant for disease diagnosis and therapy. Enzyme-linked immunosorbent assay (ELISA),26 as well as surface plasmon resonance (SPR),27 gel electrophoresis-based gelatin zymography28 had been applied to determined MMP-2, but these methods often suffers from complicate operations, costly antibody proteins and unstable antigen–antibody immunoreaction,26 especially in complex biological matrixes. Recently, methods based on hydrolytic cleavage of substrate peptides by the active protease had been reported, providing a new approach for MMP-2 detection with good selectivity due to the high specificity of the enzymatic cleavage.25
In this study, based on the fact that the polypeptide chain can be absorbed by the MOF and fluorescence of Pdots adsorbed on the MOF can be quenched quickly, a sensitive fluorescence biosensor for MMP-2 determination has been developed. A polypeptide chain (COOH–GHHYYGPLGVRGC–NH2) comprising the specific MMP-2 substrate domain (PLGVR) and π-rich motif (HHYY) was designed and linked to the surface of poly(9,9-dioctylfluorenyl-2,7-diyl) (PFO) dots. After the addition of MOF (in this study H2dtoaCu has been used as the model MOF material), the π-rich motif (HHYY, which consisted of four aromatic residues) of the polypeptide was adsorbed on the surface of MOF and led to the fluorescence quenching of PFO through the fluorescence resonance energy transfer (FRET) process. At the present of MMP-2, the peptides chain was split accurately and cause the releasing of PFO from MOF, resulting in the restoration of fluorescence of the system.
2. Experiments section
2.1 Regents and solutions
The substrate peptide ligand was synthesized by GLBiochem Ltd. (Shanghai), its sequence was:COOH–GHHYY GPLG↓VA GC–NH2
Peptide fragments: COOH–GHHYY GPLG
Bold character of the amino acid sequence is the specific recognition sequence of the matrix metalloproteinase MMP-2 and the arrow indicates the restriction site of MMP-2.
The latent formant of Matrix metalloproteinases MMP-1, MMP-2, bovine serum albumin (BSA), immunoglobulin (IgG) were purchased from Sino Biological Inc. (Beijing, China). Brij-35 was purchased from Amresco Ltd (Shanghai, China); p-aminophenylmercuric acetate (APMA) was purchased from SH-Genmed Co Ltd. (Shanghai, China); 1-ethyl-3-[(3-dimethylamino) propyl]carbodiimide (EDC), N-hydroxysuccinimide (NHS) and Tris (hydroxymethyl)aminomethane were purchased from Shanghai Medpep Co. Ltd (Shanghai, China). All aqueous solutions were prepared using ultrapure water (Mill-Q, Millipore, 18.2 MΩ resistivity).
All experiments were dissolved in TCNB Assay Buffer (pH 7.5, 50 mmol L−1 Tris, 10 mmol L−1 CaCl2, 150 mmol L−1 NaCl, 0.05% (w/v) Brij35) and stored as 4 °C for later using. 10 mM p-aminophenylmercuric acetate (APMA) was stocked in DMSO.
Substrate peptides stock solution: dissolved in dimethyl sulfoxide firstly, then diluted by the buffer solution to 10 mmol L−1 and control of dimethyl sulfoxide content lower than 1%.
Cary eclipse fluorescence spectrophotometer (Varian Corporation, USA) had been applied for the fluorescent measurements with the apparatus parameters as follows: λEx = 380 nm (slit 5 nm), λEm = 400–600 nm (slit 5 nm). Fluorescence intensity at 438 nm has been used for quantitative analysing. Each point had been detected for three times and the average value had been used.
2.2 Synthesis of the H2dtoaCu-MOF
H2dtoaCu was prepared according to the early reported procedures.18 Briefly, 5% H2dtoaH2 ethanol solution had been added into the lukewarm CuSO4 aqueous solution with stirring. Then the black precipitate was washed by water and ethanol sequent for several times, and then separated from the supernatant fraction with the centrifuge. The gelatinous precipitate was centrifugated and dried in an evacuated desiccator for the following experiment. 1.0 mg H2dtoaCu powder was dispersed into 1.0 mL double-distilled water with the assistant of oscillation and ultrasound and then stored at 4 °C for later use.2.3 Procedures for asymmetrical functionalized poly(9,9-dioctylfluorenyl-2,7-diyl) dots (PFO dots)
The PFO dots were prepared according to our early report.7 Briefly, the polymer PFO and amphiphilic copolymer PSMA were dissolved in THF to reach a stock solution of 1 mg mL−1. Then appropriate amount of polymer solutions were diluted by THF to produce a solution which contains 50 ppm PFO and 20 ppm PSMA. The mixture was sonicated and standing overnight to reach a homogeneous solution. 5 mL mixture solution was added into 10 mL of ultrapure water quickly with a vigorous bath sonicator. The remaining THF was removed by evaporation at the temperature of 74 °C followed by filtration through a 0.2 μm membrane filter. The maleic anhydride units of PSMA molecules can be hydrolyzed in the aqueous environment and generate carboxyl groups on PFO dots.Surface conjugation was performed by utilizing the EDC-catalyzed reaction between carboxyl PFO dots and the amine-modified peptide ligand. 500 μL carboxyl PFO dots solution (1 ppm), 500 μL of amine-modified peptide ligand (10 mmol L−1), 500 μL of freshly-prepared EDC solution (0.1 mol L−1) and 500 μL of NHS solution (0.1 mol L−1) were mixed in a 2 mL tube and then magnetically stirred for 4 hours at 37 °C.
2.4 Fluorescent determination procedures
The latent formant of pro-MMP-2 was activated with APMA according to the protocols provided by the manufacturer. The specific method is as follows: first, before addition to the proenzyme solution, APMA (10 mM, 3.5 mg mL−1) in assay buffer had been diluted to 1 mM. Then 1 mM APMA had been added into a pro-MMP-2 solution to activate pro-MMP-2, and the mixture was incubated in a 37 °C water bath for 1 hour with gentle shaking.The activated MMP-2 and substrate were diluted to 0.2 μg mL−1 and 20 μM, respectively, by TCNB buffer. 1 ppm peptide-linked PFO had been mixed with various concentrations of H2dtoaCu in the Eppendorf (EP) tubes firstly. Then added TCNB buffer to the above mentioned solution to reach the total volume of 200 μL. After this procedure, the mixtures were incubated at room temperature for 5 min and then subjected to the fluorescence measurement.
Finally, MMP-2 determination experiment in aqueous. In an Eppendorf (EP) tube load 100 μL of 20 μmol L−1 peptide-linked PFO, a certain amount of activated 0.2 μg mL−1 MMP-2, and the mixtures were incubated at 37 °C with gentle shaking for 2 h. The reaction mixture was then directly subjected to fluorescence measurements. In addition, we also did substrate blank experiment.
3. Results and discussion
3.1 Characterizations of PFO dots and MOFs
The surface morphology of MOFs and PFO dots were characterized with Atomic Force Microscope (AFM). MOF material is inhomogeneous with its size height of about 20 nm and grain diameter of about 200 nm (Fig. 1(a)), while the prepared PFO dots presented in a homogeneous status (Fig. 1(b)). The size distribution of PFO particles were measured and statistics analyzed by DLS, which revealed that the PFO particles possessed an average diameter of 52 nm (Fig. 1(c)).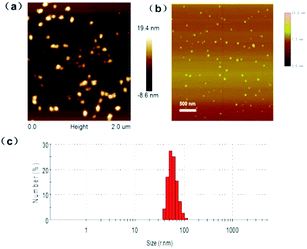 | ||
| Fig. 1 (a) The characterization of Cu–MOF and (b) PFO dots by AFM. (c) Size distribution of PFO dots. | ||
3.2 Principle of the biosensor
The main processes for analysis of MMP-2 were depicted in Scheme 1. H2dtoaCu possesses a two-dimensional sheet structure, exhibits a large surface area and conjugated p-electron system, which can interact with the peptide ligand and adsorb it firmly. The peptide ligand modified Pdots would be anchored onto the surface of H2dtoaCu in a specifically form as a result of different diameters between H2dtoaCu porthole (0.6 nm) and Pdots (52 nm), also the electrostatic repulsion between H2dtoaCu and Pdot with negatively charged. Thus, it largely decreases the non-specific FRET compared with the early reported spherical quencher.25 At the absent of the peptide chain, Pdots and H2dtoaCu keep a certain distance and give out an established fluorescence. Only when the peptide chain modified Pdots anchor to H2dtoaCu surface, the FRET (peptide chain is less than 10 nm) would led to fluorescence quenching. After the addition of target (MMP-2), peptide substrates can be specifically identified and cut by MMP-2. At this stage, only the ligands which contain two amino acids had been modified on the Pdots surface still, so the Pdots are easily remove away from the H2dtoaCu surface. Hence, the fluorescence of the system can be restored, and which can be applied to quantitative analysis of the target. In addition, as an effective luminophor, Pdots plays a key role in the ultrasensitive detection of MMP-2 in complex matrix. Combing the original properties of H2dtoaCu and Pdots, the biosensor was constructed and correlative research was studied.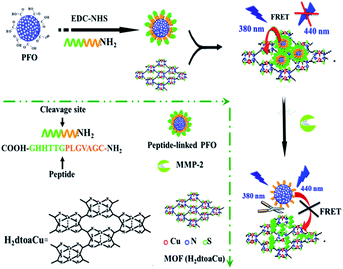 | ||
| Scheme 1 Schematic illustration of the MMP-2 biosensor based on FRET from peptide-linked PFO to H2dtoaCu. | ||
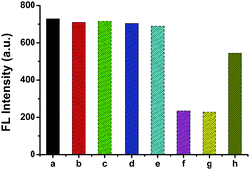
Simple contract experiments had been performed to validate the feasibility of the strategy. As shown in Fig. 2, the fluorescence intensity of the peptide modified Pdots (column b) is nearly the same with that of the Pdots (column a), this indicates that the peptide ligand cause no interference to the fluorescence of the Pdots. In addition, we compare the fluorescence intensity of the mixture comprising peptide fragments of MMP-2 and Pdots (column c) with that of the mixture of MMP-2 and peptide modified Pdots (column d), no fluorescence intensity changing had been observed also. The intensity of the mixed solution of Pdots and H2dtoaCu (column e) is the same with that of the pure Pdots solution, which means that no interaction between the two compounds occurred. But if the Pdots had been modified with peptide firstly and then mixed with H2dtoaCu, the fluorescence of the system decreases greatly (column f), the reason maybe lies in that the peptide modified Pdots had been adsorbed on H2dtoaCu surface and FRET between Pdots and H2dtoaCu occurred. The addition of the active reagent (APMA) in the above mentioned solution causes no effect on the fluorescence of the system (column g), but if MMP-2 had been added in the system shown in column f, a greatly fluorescence enhancement can be observed (column h), this means some Pdots have release back into the solution. These results demonstrate the feasibility of our proposal and preclude possible nonspecific interactions that may cause the fluorescence change.
3.3 Optimization of the reaction conditions
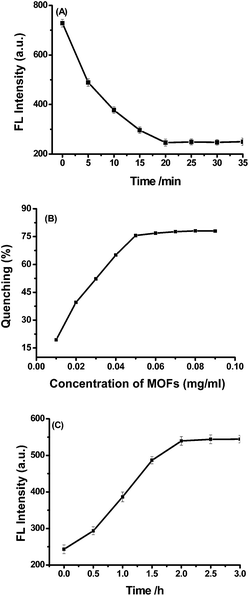
The incubation time between H2dtoaCu and the peptide modified Pdots cause great effect on the fluorescence intensity of the system. As shown in Fig. 3(A), the fluorescence intensity of the system decreased greatly after the addition of H2dtoaCu into the Pdots solution in the first 5 min, then decrease slowly with the increasing of incubation time. After 20 min, the intensity did not decrease anymore, so 20 min had been chosen as the best incubation time in the following study.The dosage of H2dtoaCu also caused effect on the fluorescence system of the system. As shown in Fig. 3(B), the concentration of peptide modified Pdots had been settled as 1 ppm, it is found that fluorescence intensity decreases gradually with the increasing of H2dtoaCu concentration, the fluorescence intensity of the system reach a plateau after 0.05 mg mL−1. This indicates that 0.05 mg mL−1 H2dtoaCu is enough to adsorb peptide modified Pdots in the solution, and at this condition, the quenching rate is 74.5%. Hence, 0.05 mg mL−1 H2dtoaCu are used to following experiment.
The fluorescent recovery has a relationship with the incubation time of complex peptide modified Pdots/H2dtoaCu and target (shown in Fig. 3(C)). Upon increasing incubation time, more adsorbed Pdots are released from H2dtoaCu, resulting in the recovery of fluorescence gradually. When the incubation time exceeds 2.0 h, the intensity no longer increases. Thus, 2.0 h is chosen as the optimized condition.
3.4 Calibration curve and detection limit
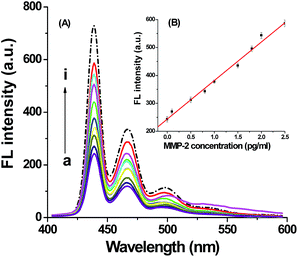
Different concentration of MMP-2 had been added into peptide modified Pdots/H2dtoaCu solution at the optimized conditions. As shown in Fig. 4(A), the fluorescence intensity increase with the increasing of MMP-2 concentrations. And the fluorescence intensity had a direct relationship with MMP-2 concentration in the range of 0.1–2.5 pg mL−1 (as shown in Fig. 4(B), each point was detected for 5 times and the average values were used), the linear regression equation was:| I = 138.1C + 243.2 R = 0.9941 |
In addition, five Pdots/H2dtoaCu solutions were prepared and used to detect 2.0 pg mL−1 MMP-2 as a parallel experiment, the relative standard deviation (RSD) was 3.34%, this indicated the proposed method had good repeatability. If the five Pdots/H2dtoaCu solutions were prepared and every week took out one for testing (at 2.0 pg mL−1 MMP-2), the RSD was 4.54%, this means the prepared solution had good stability also.
3.5 Selectivity and interference study
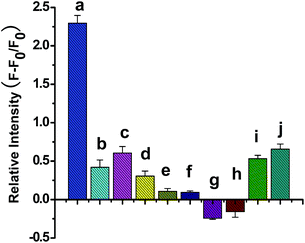
The specificity of the proposed biosensor was studied by choosing MMP-1, some metal ions, amino acids and proteins as interferents. The concentration of MMP-2 was set as 0.2 ng mL−1, the concentration of interferent of MMP-1 was 1.0 ng mL−1 and the concentrations of the other interferents were 0.1 μmol L−1. As shown in Fig. 5, there was a remarkable signal increase at the presence of MMP-2, while there was minimal change with respect to interferents. These results indicated that owing to the high specificity of the enzymatic cleavage, MMP-2 can be quantificationally detected in complex matrix without interferences.4. Conclusion
In conclusion, we have designed a novel biosensor for sensitive and quantitative detection of MMP-2 based on fluorescence resonance energy transfer between the peptide-linked PFO dots and MOF. The peptide-linked PFO dots can be adsorbed by the H2dtoaCu and the fluorescence of PFO dots can be quenched subsequently. The addition of the target causes the releasing of PFO from MOF and the restoration of the fluorescence of the system. MMP-2 can be quantificationally detected with high sensitivity owing to the ultrabright luminophor of PFO dots. Furthermore, since substrate peptide can be split by MMP-2 specifically, great selectivity was provided. This assay offered great potential for rapid, simple and sensitive analysis of other biomolecules.Acknowledgements
This project was financially supported by the National Basic Research Program of China (no. 2010CB732403), National Nature Sciences Funding of China (21175024, 21222506, 21375021), program for New Century Excellent Talents in University (NCET-12-0619) and the Natural Sciences Funding of Fujian Province (2012J06005, 2014J06005).Notes and references
- C. F. Wu, Y. H. Jin, T. Schneider, D. R. Burnham, P. B. Smith and D. T. Chiu, Angew. Chem., Int. Ed., 2010, 49, 9436–9440 CrossRef CAS PubMed
.
- C. F. Wu, B. Bull, C. Szymanski, K. Christensen and J. McNeill, ACS Nano, 2008, 2, 2415–2423 CrossRef CAS PubMed
.
- J. Pecher and S. Mecking, Chem. Rev., 2010, 110, 6260–6279 CrossRef CAS PubMed
.
- C. F. Wu, T. Schneider, M. Zeigler, J. B. Yu, P. G. Schiro, D. R. Burnham, J. D. McNeill and D. T. Chiu, J. Am. Chem. Soc., 2010, 132, 15410–15417 CrossRef CAS PubMed
.
- F. Xia, X. L. Zuo, R. Q. Yang, Y. Xiao, D. Kang, A. Vallee-Belisle, X. Gong, A. J. Heeger and K. W. Plaxco, J. Am. Chem. Soc., 2010, 132, 1252–1254 CrossRef CAS PubMed
.
- H. A. Ho, A. Najari and M. Leclerc, Acc. Chem. Res., 2008, 41, 168–178 CrossRef CAS PubMed
.
- Z. Y. Lin, G. Y. Zhang, W. Q. Yang, B. Qiu and G. N. Chen, Chem. Commun., 2012, 48, 9918–9920 RSC
.
- P. Horcajada, R. Gref, T. Baati, P. K. Allan, G. Maurin and P. Couvreur, Chem. Rev., 2012, 112, 1232–1268 CrossRef CAS PubMed
.
- C. A. Bauer, T. V. Timofeeva, T. B. Settersten, B. D. Patterson, V. H. Liu and B. A. Simmons, J. Am. Chem. Soc., 2007, 129, 7136–7144 CrossRef CAS PubMed
.
- L. J. Murray, M. Dinca and J. R. Long, Chem. Soc. Rev., 2009, 38, 1294–1314 RSC
.
- J. R. Li, Y. Ma, M. C. McCarthy, J. Y. Sculley, H. K. J. Jeong, P. B. Balbuena and H. C. Zhou, Coord. Chem. Rev., 2011, 255, 1791–1823 CrossRef CAS PubMed
.
- A. Dhakshinamoorthy, M. Opanasenko, J. Čejka and H. Garcia, Catal. Sci. Technol., 2013, 3, 2509–2540 CAS
.
- C. X. Yang and X. P. Yan, Anal. Chem., 2011, 83, 7144–7150 CrossRef CAS PubMed
.
- Z. Y. Gu, G. Wang and X. P. Yan, Anal. Chem., 2010, 82, 1365–1370 CrossRef CAS PubMed
.
- P. Horcajada, C. Serre, G. Maurin, N. A. Ramsahye, F. Balas, M. Vallet-Regi, M. Sebban, F. Taulelle and G. Ferey, J. Am. Chem. Soc., 2008, 130, 6774–6780 CrossRef CAS PubMed
.
- G. Lu and J. T. Hupp, J. Am. Chem. Soc., 2010, 132, 7832–7833 CrossRef CAS PubMed
.
- L. E. Kreno, J. T. Hupp and R. P. V. Duyne, Anal. Chem., 2010, 82, 8042–8046 CrossRef CAS PubMed
.
- L. F. Chen, H. Y. Zheng, X. Zhu, Z. Y. Lin, L. H. Guo, B. Qiu, G. N. Chen and Z. N. Chen, Analyst, 2013, 138, 3490–3493 RSC
.
- X. Zhu, H. Y. Zheng, X. F. Wei, Z. Y. Lin, L. H. Guo, B. Qiu and G. N. Chen, Chem. Commun., 2013, 49, 1276–1278 RSC
.
- T. Myochin, K. Hanaoka, T. Komatsu, T. Terai and T. Nagano, J. Am. Chem. Soc., 2012, 134, 13730–13737 CrossRef CAS PubMed
.
- W. J. Akers, B. G. Xu, H. Lee, G. P. Sudlow, G. B. Fields, S. Achilefu and W. B. Edwards, Bioconjugate Chem., 2012, 23, 656–663 CrossRef CAS PubMed
.
- V. Quesada, G. R. Ordonez and L. M. Sanchez, Nucleic Acids Res., 2009, 37, 239–243 CrossRef PubMed
.
- M. Egeblad and Z. Werb, Nat. Rev. Cancer, 2002, 2, 161–174 CrossRef CAS PubMed
.
- J. M. Fang, Y. Shing, D. Wiederschain, L. Yan, C. Butterfield, G. Jackson, J. Harper, G. Tamvakopoulos and M. A. Mose, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 3884–3889 CrossRef CAS
.
- Y. H. Wang, P. Shen, C. Y. Li, Y. Y. Wang and Z. H. Liu, Anal. Chem., 2012, 84, 1466–1473 CrossRef CAS PubMed
.
- S. Patel, G. Sumitra, B. C. Koner and A. Saxena, Clin. Biochem., 2011, 44, 869–872 CrossRef CAS PubMed
.
- U. Pieper-Fürst, U. Kleuser, W. F. M. Stöcklein, A. Warsinke and F. W. Scheller, Anal. Biochem., 2004, 332, 160–167 CrossRef PubMed
.
- T. Yamane, M. Mitsumanta, N. Yamaguchi, T. Nakazawa, K. Mochizuki, T. Kondo, T. Kawasaki, S. Murata, Y. Yoshida and R. Katoh, Cell Tissue Res., 2010, 340, 471–479 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2014 |