Effective near-infrared absorbent: ammonium tungsten bronze nanocubes†
Mei Yan,
Hongxi Gu,
Zhouzhou Liu,
Chongshen Guo* and
Shaoqin Liu*
Key Laboratory of Microsystems and Micronanostructures Manufacturing (Ministry of Education), Harbin Institute of Technology, Harbin 150080, China. E-mail: shaoqinliu@hit.edu.cn; chongshenguo@hit.edu.cn
First published on 14th November 2014
Abstract
Hardly any other compound has realized better optical absorption of near-infrared (NIR) rays (780–2500 nm) than tungsten bronze nanoparticles in terms of absorption coefficient, widths of the working spectrum, photothermal transformation efficiency and their own physicochemical stability. However, efforts concerning the development of tungsten bronze nanoparticles for serving as a NIR absorbent are very limited due to the shortage of effective approaches to obtain these nanoparticles, especially for tungsten bronzes with insertion of bigger cations, such as CsxWO3 and (NH4)xWO3. In this work, we describe how to fabricate (NH4)xWO3 using a high-temperature but short-time solvothermal process, which involves employing oleic acid–oleylamine as the solvent and WCl6 as the W resource, together with the inspection of its NIR-absorption related properties. The nanocubes of 100 nm have been characterized by XRD, TG-MS, XPS and TEM to examine the crystal phase and nanostructures. Moreover, the dispersion of the nanocubes in the form of a thin film was used to investigate the NIR absorption properties. As determined by the optical test, the thin film consisting of the nanocubes exhibits extraordinary features as a solar control window, which can transmit the majority of visible light while absorbing nearly all of the NIR rays from 780 nm to 2500 nm. Meanwhile, the (NH4)xWO3 thin film can maintain its high shielding effect for the 1064 nm NIR light up to 35.3 kW m−2 radiation and has excellent cyclic stability for 100 cycles without obvious optical changes. Finally, it has been found that the (NH4)xWO3 nanocubes show a remarkable photothermal conversion phenomenon even when dispersed in a thin film.
1. Introduction
Near-infrared (NIR) light refers to radiation with a wavelength ranging from 780 to 2500 nm, which makes up to 52% of the photo-energy of sunlight and is often called a heat ray due to being perceived by the human body as heat.1 Actually, the near-infrared region is a burgeoning and unique optical area where the transmittance of radiation is rarely interfered with under natural conditions since there are a lack of natural NIR absorbents. The development of an effective NIR absorbent with a broad working waveband is an advanced hot topic which has significant meaning for the improvement of the living conditions of human beings from the viewpoint of photothermal ablation, energy economization, solar collectors, heat-ray shielding smart windows, stealth technology, and optical filters.2Over the past few decades, it has been well known that organic dyes,3 metal complexes with organic ligands,3 lanthanide boride4,5 and noble metal nanoparticles6–13 can realize photoabsorption in the NIR region. However, each of them has their own drawbacks. Optical absorption around 600–900 nm originates from transitions between different energy levels in the molecule for these organic dyes and metal complexes with organic ligands, however, the low coefficient of light absorption together with severe photobleaching limit them becoming outstanding candidates in this area.3 Hexaboride nanoparticles absorb only a certain extent of the wavelengths of NIR rays and are not effective for all NIR rays.4,5 Moreover, a reductive atmosphere and a high temperature (ca. 1500 °C) are necessary for the syntheses of bulk hexaborides. Afterwards, it exhausts much energy to crush these microsized grains into small nanoparticles because of their high hardness. As for the variation of surface plasmon resonance, the optical absorption band of noble metal nanoparticles can be readily tuned in the region of 500–880 nm by controlling the shape, size or aspect ratios.7–13 Despite this advance, the development of an applicable noble-metal-based NIR absorbent is hurdled by high price, poor scalability and difficulty in obtaining a stable powder sample. Additionally, all of the aforementioned compounds only achieve absorption of certain frequencies of NIR light, rather than exhibiting strong photoabsorption across the whole NIR region of 780–2500 nm. Therefore, it is extremely urgent to develop an effective NIR absorbent with a broad working waveband, especially for the exploitation of oxide-based nanomaterials which show much better physicochemical stability, more natural abundance and ease of operation. A WO3-based electrochromic film seems likely to be an option regarding the thermal management of windows, but it mainly controls the transmittance of visible light through coloring/decoloring cycles instead of controlling the NIR, leading to a lower inner brightness on the coloring state.
Our previous research confirmed that, when dispersed as nanosized particles, hexagonal tungsten bronze type compounds (MxWO3, M = Cs, K, Na, Rb, NH4+) consisting of mixed chemical valence tungsten ions (W6+ and W5+) exhibited excellent NIR absorption properties in a wide range of 780–15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 nm, covering the whole waveband of the NIR region and part of the mid-IR area.14 Among these bronzes, (NH4)xWO3 is seldom investigated, which may be related to the great synthetic difficulty originating from the high structural distortion of (NH4)xWO3 as a result of the insertion of big NH4+ ions into the WO6 octahedral framework. The designed growth of (NH4)xWO3 nanoparticles is of great significance as they also consist of mixed valence tungsten ions and are expected to show NIR shielding ability as well. Furthermore, on the merits of its open-tunnel structure and special electronic structure, the mobility of the cations in the channels of the WO3 framework allows one to dramatically modify the electronic properties by ion exchange or intercalation, giving the tungsten bronze of (NH4)xWO3 a wide range of related applications as catalytic, battery, gas sensing, and electrochromic materials.15 Previously, Szilágyi and coworkers synthesized the (NH4)xWO3 nanoparticles by heating APT in H2 and confirmed that the decomposition temperature for (NH4)xWO3 was about 550 °C.16 More recently, colloidal nanocrystals of CsxWO3 were fabricated by Mattox et al. via employing oleic acid–oleylamine as a solvent at 300 °C.17 Inspired by the above reports, in this work, we synthesized (NH4)xWO3 nanocubes under an oleic acid–oleylamine medium at a selected temperature of 350 °C, which is lower than the decomposition temperature of (NH4)xWO3 but high enough for rapid fabrication of the nanocrystals.
000 nm, covering the whole waveband of the NIR region and part of the mid-IR area.14 Among these bronzes, (NH4)xWO3 is seldom investigated, which may be related to the great synthetic difficulty originating from the high structural distortion of (NH4)xWO3 as a result of the insertion of big NH4+ ions into the WO6 octahedral framework. The designed growth of (NH4)xWO3 nanoparticles is of great significance as they also consist of mixed valence tungsten ions and are expected to show NIR shielding ability as well. Furthermore, on the merits of its open-tunnel structure and special electronic structure, the mobility of the cations in the channels of the WO3 framework allows one to dramatically modify the electronic properties by ion exchange or intercalation, giving the tungsten bronze of (NH4)xWO3 a wide range of related applications as catalytic, battery, gas sensing, and electrochromic materials.15 Previously, Szilágyi and coworkers synthesized the (NH4)xWO3 nanoparticles by heating APT in H2 and confirmed that the decomposition temperature for (NH4)xWO3 was about 550 °C.16 More recently, colloidal nanocrystals of CsxWO3 were fabricated by Mattox et al. via employing oleic acid–oleylamine as a solvent at 300 °C.17 Inspired by the above reports, in this work, we synthesized (NH4)xWO3 nanocubes under an oleic acid–oleylamine medium at a selected temperature of 350 °C, which is lower than the decomposition temperature of (NH4)xWO3 but high enough for rapid fabrication of the nanocrystals.
2. Experimental
2.1 Synthesis of (NH4)xWO3 nanocubes
Under standard conditions, first, 0.1 g WCl6 was dissolved into 8 ml of oleic acid to form a yellow solution, to which 2 ml of oleylamine was added. Then, the resulting solution was transferred into a batch reactor (Fig. S1†) with a 20 ml internal volume, and the reactor was sealed and placed in a block heater, which had been preheated to 350 °C, to start the solvothermal reaction. During the heating, the reactors underwent shaking on a reciprocating shaker at a frequency of 50 times per min. After reaction for 1 h, the precipitate was collected by centrifugation, carefully washed with distilled water and ethanol, and dried at 60 °C under vacuum overnight to get the final product (the rinsing of the precipitates with water is a dispensable step, but there is a negligible effect of water-rinsing on the NIR absorptive performance).2.2 Characterization
The phase composition of the sample was determined by X-ray diffraction analysis (XRD, Shimadzu XD-1) using graphite-monochromized CuKα radiation. The size and shape of the nanoparticles were observed by transmission electron microscopy (TEM, JEOLJEM-2010). HRTEM images and SAED images were obtained on a ZEISS LEO 922 with an accelerating voltage of 200 kV. The surface composition of the samples and the binding energy of W4f were determined by X-ray photoelectron spectroscopy (XPS, Perkin Elmer PHI 5600). XPS data have been calibrated using the C1s line. The thermogravimetric and differential thermal analyses (TG-DTA, riggaku, TG8101D) were performed for the sample from room temperature to 900 °C at a heating rate of 10 °C min−1 in N2. Atomic force microscopy (AFM, NPX100) measurements were performed for observing the dispersion state of the nanoparticles on the film. The surface charge status of the nanorods was examined by zeta-potential measurements using a Nanosizer (Malvern Instruments Ltd., Nano-ZS).2.3 Optical test
To evaluate the NIR absorption characteristics of the nanocube-containing thin film, (NH4)xWO3 powder was dispersed in a collodion–ethanol mixed solution at a mass ratio of ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) collodion
collodion![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) powder = 1.0
powder = 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.93
0.93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.15 (the collodion used in this work contains 10 wt% of cellulose nitrate and it was purchased from KANTO CHEMICAL CO. INC, Japan). Then, the coating solution was painted onto a quartz glass, by an applicator, with a concave depth of 12.5 μm. The optical response of the coating was measured using a spectrophotometer (JASCO V-670), giving an output of transmittance in the UV, visible, and infrared ranges (200–2700 nm).
0.15 (the collodion used in this work contains 10 wt% of cellulose nitrate and it was purchased from KANTO CHEMICAL CO. INC, Japan). Then, the coating solution was painted onto a quartz glass, by an applicator, with a concave depth of 12.5 μm. The optical response of the coating was measured using a spectrophotometer (JASCO V-670), giving an output of transmittance in the UV, visible, and infrared ranges (200–2700 nm).
We selected a 1064 nm laser as the light source and carefully examined the photothermal effect and optical shielding properties of the (NH4)xWO3 thin film. NIR laser irradiation induced temperature elevation was recorded using a thermographic meter (FLIR System i7).
3. Results and discussion
As shown in the TEM image (Fig. 1a), the obtained sample mainly consists of many randomly-distributed rectangular nanoparticles with smooth and dense surfaces. If we carefully observe the deckle edges of the nanocubes, these particles are likely composed of many tiny bundles of nanofibers. In addition, the aggregation of several nanocubes into big congeries has been observed. The particle size distribution reveals a wide size distribution from 40 nm to hundreds of nm, however, the most probable value is around 90 nm (Fig. 1a inset), being in agreement with the observation of the TEM image. The HR-TEM image of a certain select part of a single nanocube, shown in Fig. 1b, depicts clear lattice fringes, suggesting that the nanocubes are well crystallized under heating at 350 °C. In addition, the crystalline lattice constant along the nanocube was calculated as 0.378 nm, which agrees well with the interplanar spacing (0 0 2) of the desired (NH4)0.33WO3. To validate this, XRD analysis has been further employed to identify the phase composition and crystallographic structure of the sample. Fig. 1c shows the XRD pattern of the as-obtained blue (NH4)xWO3 powder, together with the standard powder diffraction pattern of (NH4)0.33WO3 as a reference (JCPDS card no. 42-0452). All of the reflections shown in Fig. 1c could be well indexed as the hexagonal ammonium tungsten bronze with known crystal lattice parameters of a = 0.7392 nm and c = 0.7512 nm, and no characteristic peaks for impurities such as WO3 or WO3−x were observed. For the hexagonal tungsten bronzes (MxWO3, x ≤ 0.33), the structure mainly comprises a rigid tungsten–oxygen framework built up of layers containing corner-sharing WO6 octahedron, which are arranged in six-membered rings. The layers are stacked along the c-axis, leading to the formation of one-dimensional open hexagonal channels, which are occupied randomly by cations (Fig. 1d).18 Intercalation of ammonium ions into these channels doubtlessly causes great architectural distortion and tilt, leading to a decrement in the orderliness of the crystal unit. This explains why less successful cases were known to obtain (NH4)xWO3 with uniform size or morphology. Next, we carefully examined the chemical composition and chemical state of the (NH4)xWO3 nanocrystals by X-ray photoelectron spectroscopy (XPS). A complex energy distribution of the W4f photoelectrons was obtained as shown in Fig. 1e. After deconvolution, the W4f core-level spectrum could be well fitted into two groups of spin–orbit doublets, corresponding to two different oxidation states of W atoms. The main peaks, giving W4f 5/2 at 37.7 eV and W4f 7/2 at 35.6 eV, could be attributed to the W atoms being in a 6+ oxidation state. The second doublet, with lower binding energies of 34.4 eV and 36.5 eV, could be assigned to the emission of W4f 5/2 and W4f 7/2 core levels from atoms in an oxidation state of 5+. These results on the core level of the tungsten ions are in good agreement with the typical nature of tungsten bronze (MxW6+1−xW5+xWO3). Although the deconvolution of the XPS spectra showed only the presence of W5+ and W6+ in the sample, we can not exclude the presence of a trace amount of W4+. Actually, in the study by Szilágyi et al. it was found that there was a small amount of W4+ in the reduced tungsten bronze nanoparticles. Due to the presence of W5+, the color of the nanocube is dark blue.16 Other than tungsten, the elements N, O and C (Fig. S2†) have been found in the sample as well. The presence of carbon in the final product may be due to the residual chemically or physically adsorbed organic debris originating from the solvent molecules, while the N comes from the NH4+ in the sample. The chemical composition as determined by deconvolution of the XPS spectrum is (NH4)0.25WO3.The thermal behaviour of the (NH4)xWO3 nanocubes was investigated via thermogravimetry (TG) measurements at a heating rate of 10 °C min−1, meanwhile, the resulting gas produced during the heating was directed into the mass spectrum (MS) analyzer to analyse its chemical identity. As shown in Fig. S3,† there are two stages of obvious weight loss, attributed to the desorption of water (∼230 °C) and the departure of NH3 from the sample as a result of (NH4)xWO3 decomposition (230–515 °C). This is evidenced by the results of mass spectrometry in which mass-to-charge (m/z, where m is the mass of the ion, and z is the ion charge) ratios of 17, 18 and 28 (Fig. 4b), corresponding to the chemical species of NH3, H2O, and N2, respectively, were found. The observation of (NH4)xWO3 decomposition and the detection of NH3 departure by the TG-MS method were also observed in other’s work.15 According to Szilágyi’s report,19,20 the weight loss at 250–550 °C in the TG curve is due to the departure of both NH3 and NH4+, located at the inner site of the sample. By calculating the weight loss in this range, we can easily obtain the total amount of NH3 and NH4+ present in the nanocube. On the other hand, the content of NH4+ could be obtained from XPS spectra. This is because each NH4+ ion contributes one electron to the tungsten conduction band, leading to the formation of one W5+. In this way, the chemical formula of the nanocube could be expressed as (NH3)0.05 (NH4)0.25WO3 and the ratio of NH4+/NH3 is 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
On the basis of the aforementioned XRD, XPS and TG results, the obtained nanocubes have a well proven phase nature of (NH4)xWO3.
The experimental parameters which may influence the crystalline structure of the nanoparticle have also been varied to obtain a clear understanding of the crystal growth process under such a reaction system. To clearly validate the role of oleylamine, a series of control experiments with different contents of oleylamine was conducted under the same reaction temperature and time period, as well as keeping the total volume of mixed oleylamine–oleic acid solution as 10 ml. When 1 ml of oleylamine was added into the reaction medium, the sample was observed as a mixture of small nanocubes and bundles of nanofibers (Fig. S4†). In contrast, homogeneous oblong blocks with a glossy appearance could be distinguished for the sample synthesized with the assistance of 5 ml oleylamine (Fig. 5b). Although the morphologies of the samples synthesized with 2 or 5 ml oleylamine are very similar to each other (Fig. S4†), the yields obtained have drastic differences relative to the content of oleylamine, that is, excessive introduction of oleylamine results in a decline in production. This is due to a remarkable pH increment as a result of over-adding oleylamine, which obstructs the formation of the powder. Therefore, sufficiently acidic conditions are necessary for the condensation of dissociated tungsten ions in the solvent into the final tungsten oxide solid. Everything has two sides, just like a coin – in the absence of oleylamine we were unable to produce a definite phase of ammonium tungsten bronze, instead, an unknown phase of product was obtained for the sample synthesized in pure oleic acid solution (Fig. S5†). Apart from as a pH modifier, oleylamine also has been considered as a capping agent that plays a vital role in tailoring the nanostructure of the nanoparticles. For the (NH4)xWO3, we propose that the morphology of the sample depends on the relative growth rates along the c-axis (νc) and the lateral side (νL) to a great extent. In virtue of possessing one-dimensional hexagonal channels along the c-axis (Fig. 1d), the fastest growth rate is achieved by linking WO6 octahedra to form these channels along the c-axis, and this leads to the tungsten bronzes being preferentially grown along the c-axis (Fig. S4c† upside).14 Therefore, tungsten bronze nanomaterials have been intensively reported in the one-dimensional form, such as microrods and microfibers, where the growth rate along the c-axis is dominating over that of the lateral side.14 In this work, it has been considered that selective absorbing of the oleylamine molecules onto crystallographic facets exposed along the c-axis slows down the growth rate of νc to the degree of being almost the same as νL, and this results in equivalent growth along both the c-axis and lateral directions, generating homogenous (NH4)xWO3 nanocubes (Fig. S4c†). Hence, it is expected that when a limited content of oleylamine is present in the reaction system the number of oleylamine molecules is not sufficient to cover all of the active sites located on every particle and this leaves part of the particles to grow into nanofibers freely (Fig. S4a†).
In addition to altering the content of the solvent, the effects of the reaction time period were investigated as well. In fact, the reaction time period has negligible influence on the nanostructure, and the samples obtained after 0.5, 1 and 2 h reactions show almost the same size and shape (Fig. S6†), whereas the yield of powder was found to increase from 30% for 0.5 h to above 90% for 1 or 2 h.
One of the interesting applications for the NIR absorbent is in energy-saving windows, which should not only shield off the NIR light to prevent incrementation of the indoor temperature under strong solar radiation in summer but also act as a heat-insulator to prevent heat-flow escaping from the room in winter. Resultantly, this could reduce energy consumption for air conditioning and thereby decrease emissions of carbon dioxide. Besides the NIR absorption characteristics, a high visible light transparency is also highly required for these windows to ensure indoor brightness. In this respect, the optical response of NIR absorbents is often measured in the form of a thin film, to directly provide observation of the transparency for visible light and absorptive capacity for NIR irradiation. In this study, the powder sample was mixed with an optically transparent binder and coated on a substrate of quartz glass. It merits special attention that the blue coating consists of just 7.7 wt% of (NH4)xWO3 nanocubes, while the remaining part is polymer binder. As shown in Fig. 2a, the coated film is tinted blue, but is highly transparent. For investigating the dispersion state of the (NH4)xWO3 nanoparticles on the surface of the film, an AFM technique was performed to provide more details on the coated film. As shown in Fig. 2b, the 2D AFM image clearly reveals that the surface layer of film is covered homogenously by many flocculent globoids with diameters in the range of 0.5–1 μm, which is much bigger than the size of the nanocube of 100 nm shown in Fig. 1a, indicating that the globoids result from the aggregation of nanoparticles and binder. The surface layer of the film is uniform and smooth, as shown in Fig. 2c, with a mean interface roughness (Ra) of 44.2 nm. In addition, the thickness of the tinted film was around 1 μm.
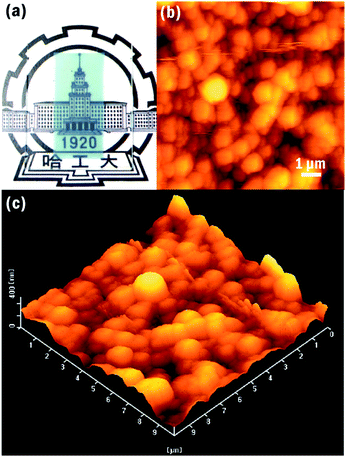 | ||
| Fig. 2 (a) Photograph of the thin film with a dispersion of (NH4)xWO3 nanocubes; (b) 2D and (c) 3D AFM images of the film coated on the quartz. | ||
Fig. 3 exhibits the transmittance spectra of the thin film composed of (NH4)xWO3 nanocubes. The NIR absorption properties are closely related to the free electrons originating from W5+ or W4+.14 For comparison, we also tested the optical performance of h-WO3 showing similar hexagonal channels to (NH4)xWO3 but differing in its fully oxidized state and low occupancy, and (NH4)xWO3+x/2 only differing in its fully oxidized state ((NH4)xWO3+x/2 was synthesized according to ref. 21). Apparently, the relevant homologues of hexagonal h-WO3 and (NH4)xWO3+x/2 are transparent for NIR irradiation and visible light (Fig. 3a and b), indicating that there is no obvious absorption abilities for these two species. The commercial Cs0.33WO3, possessing a similar chemical composition and valence to the (NH4)xWO3 nanocubes of this work, was treated under the same conditions. It can be seen that the thin film consisting of commercial microsized Cs0.33WO3 can shield off about 50% of NIR light (Fig. 3d). In sharp contrast, as shown in Fig. 3c, the (NH4)xWO3 nanocube coated film selectively transmits the majority of visible light in the range of 400–800 nm, as well as cutting off near-infrared and UV light effectively. The transmittance of NIR light is lower than 20%. Meanwhile, the reflectance characters shown in Fig. 3d clearly reveal that light scattering on the thin film is very limited in the whole region of 200–2700 nm. For the optical response of the thin film, 100% − T − R is the photo-absorptivity, where T is the transmittance of light and R is the reflectance that occurred on the surface of the coating. In this work, the low reflectance and transmittance in the NIR region for the (NH4)xWO3 nanocubes of this work manifest their outstanding NIR absorption ability. The luminous efficiency function describes the average spectral sensitivity of the human visual perception of brightness according to light of different wavelengths (black area). It can be seen that the maximum transmittance of the (NH4)xWO3 film in the visible region overlaps with the area of the luminous efficiency function, suggesting that the film can selectively transmit the more bright visible light among the visible spectrum.
On the basis of the above results, one can conclude that for realization of the NIR absorption the low valence state of W5+ and a smaller size are highly required. This can well explain why the (NH4)xWO3+x/2 consisting of W6+ only did not show any NIR absorption character and why Cs0.33WO3 with W5+ but a microsize showed weaker NIR absorption.
In addition, we also measured the optical absorbance of a powder sample in order to give direct judgment on the intrinsic optical absorption ability. It was further revealed that the (NH4)xWO3 blue powder can strongly absorb NIR light from 780 nm to 2500 nm (Fig. S8†).
For further investigation of the feasibility of employing a (NH4)xWO3 film as a NIR resistant coating, we tested its shielding properties for 100 cycles at selected power densities of 442.32 W m−2 and 4423.2 W m−2, which are close to the density of NIR light contained in sunlight and tenfold to the power of NIR light contained in sunlight, respectively. The percent transmittancy in the presence or absence of the (NH4)xWO3 film as well as the related time response under the two powder densities are shown in Fig. 4. The optical transmittance decreases from 100% to 20% under 442.32 W m−2 irradiation and to 25% under 4423.2 W m−2 irradiation (corresponding to a shielding of 75–80% of the NIR light) as soon as the film was set on the light path (within 2 seconds) and the transmittance then recovers as soon as the film is removed. Notably, a high cyclic stability was observed for the two powder densities, as the transmittance switching responses for the first 10 and last 10 cycles are nearly the same and no obvious optical change appeared. These positive results stimulated us to investigate the cyclic stability at even higher NIR laser irradiations. By successively stepping up the input power density of the NIR light, one can find that the (NH4)xWO3 film can maintain a stable shielding percentage of the NIR light up to 35.3 kW m−2 disregarding the powder density, (Fig. S9†) and that destruction of the polymer binder part of the film happened when the density overstepped this limitation. In brief, the (NH4)xWO3 film exhibits desirable photostability on the merit of its oxide nature.
During the test, we also found that there was an obvious photo-thermal conversion effect on the irradiation area of the film. As is shown in Fig. 5, the temperature of the central irradiation area, monitored by thermographic survey, increases from an ambient 24 °C to 47.6, 54.6 and 70 °C, after 5, 15 and 30 seconds exposure to the 1064 nm laser, respectively. Meanwhile, as a result of thermal diffusion from the centre, the area of the hot zone becomes larger as the increment in radiation duration occurs.
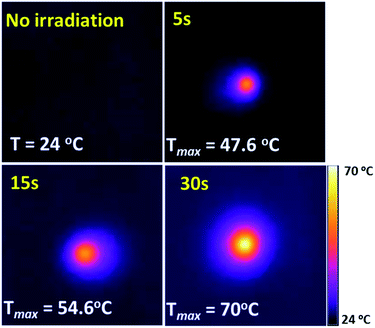 | ||
| Fig. 5 The temperature variation of the (NH4)xWO3 film with varying radiation duration (the film was irradiated by a 1064 nm laser at a power density of 17.7 kW m−2). | ||
4. Conclusions
In summary, we have presented a new one-step strategy to obtain an ammonium tungsten bronze that has never before been fabricated in the form of nanocubes. Characterization results suggested that the obtained sample was a pure phase of tungsten bronze with a mixed chemical valence of W5+ and W6+. The optical tests revealed that the (NH4)xWO3 nanocubes can strongly absorb NIR light from 780 to 2500 nm, either as a powder or when dispersed as a thin film. Remarkably, the thin film of (NH4)xWO3 nanocubes showed excellent cyclic stability even after 100 cycles and an efficient NIR shielding performance against nearly a 90-fold density of the NIR light contained in sunlight. Due to the high efficiency of absorption of NIR light, the (NH4)xWO3 nanocubes also exhibited instantaneous opt-thermal conversion upon NIR irradiation. Therefore, the resulting (NH4)xWO3 nanocubes are anticipated to be an upcoming potent candidate as a NIR absorbent with a wide response range, high absorptivity and cyclic stability.Acknowledgements
Financial support from the National Basic Research Program of China (2013CB932704), National Natural Science Foundation of China (Grant nos 21303033, 81373359, 91023007 and 20773033), New Century Excellent Talents in University, and Outstanding Young Funding of Heilongjiang Province is gratefully acknowledged. This work is also supported by “the Fundamental research Funds for the Central Universities” (Grant no. HIT. NSRIF.2015061&2015062), Heilngjiang Postdoctoral Financial Assistance (Grant no. LBH-Z13079) and China Postdoctoral Science Foundation Funded Project (Project no. 2014M551232&2014M551224).Notes and references
- C. S. Guo, S. Yin, L. J. Huang, L. Yang and T. Sato, Chem. Commun., 2011, 47, 8853 RSC
.
- C. S. Guo, S. Yin, Y. F. Huang, Q. Dong and T. Sato, Langmuir, 2011, 27, 12172 CrossRef CAS PubMed
.
- J. Faban, Chem. Rev., 1992, 92, 1197 CrossRef
.
- H. Takeda and K. Adachi, J. Am. Ceram. Soc., 2007, 90, 4059 CAS
.
- K. Adachi and M. Miratsu, J. Mater. Res., 2010, 25, 3 CrossRef
.
- K. W. Hu, T. M. Liu, K. Y. Chung, K. S. Huang, C. T. Hsieh, C. K. Sun and C. S. Yeh, J. Am. Chem. Soc., 2009, 131, 14186 CrossRef CAS PubMed
.
- B. Jang, J. Y. Park, C. H. Tung, I. H. Kim and Y. Choi, ACS Nano, 2011, 5, 1086 CrossRef CAS PubMed
.
- H. C. Huang, K. Rege and J. J. Heys, ACS Nano, 2010, 4, 2892 CrossRef CAS PubMed
.
- Z. J. Zhang, L. M. Wang, J. Wang, X. M. Jiang, X. H. Li, Z. J. Hu, Y. H. Ji, X. C. Wu and C. Y. Chen, Adv. Mater., 2012, 24, 1418 CrossRef CAS PubMed
.
- L. R. Hirsch, R. J. Stafford, J. A. Bankson, S. R. Sershen, B. Rivera, R. E. Price, J. D. Hazle, N. J. Halas and J. L. West, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 13549 CrossRef CAS PubMed
.
- L. Gao, J. B. Fei, J. Zhao, H. Li, Y. Cui and J. B. Li, ACS Nano, 2012, 6, 8030 CrossRef CAS PubMed
.
- Y. N. Xia, W. Y. Li, C. M. Cobley, J. Y. Chen, X. H. Xia, Q. Zhang, M. X. Yang, E. C. Cho and P. K. Brown, Acc. Chem. Res., 2011, 44, 914 CrossRef CAS PubMed
.
- X. Huang, S. Tang, X. Mu, Y. Dai, G. Chen, Z. Zhou, F. Ruan, Z. Yang and N. Zheng, Nat. Nanotechnol., 2011, 6, 28 CrossRef CAS PubMed
.
- C. S. Guo, S. Yin, P. L. Zhang, M. Yan, K. Adachi, T. Chonan and T. Sato, J. Mater. Chem., 2010, 20, 8227 RSC
; C. S. Guo, S. Yin, M. Yan and T. Sato, J. Mater. Chem., 2011, 21, 5099 RSC
; C. S. Guo, S. Yin and T. Sato, Nanosci. Nanotechnol. Lett., 2011, 3, 413 CrossRef CAS PubMed
; C. S. Guo, S. Yin, L. J. Huang and T. Sato, ACS Appl. Mater. Interfaces, 2011, 3, 2794 Search PubMed
; C. S. Guo, S. Yin, Q. Dong and T. Sato, CrystEngComm, 2012, 14, 7727 RSC
.
- C. S. Guo, S. Yin and T. Sato, Rev. Adv. Sci. Eng., 2012, 1, 235 CrossRef PubMed
.
- I. M. Szilágyi, J. Madarász, G. Pokol, P. Király, G. Tárkányi, S. Saukko, J. Mizsei, A. L. Tóth, A. Szabó and K. V. Josepovits, Chem. Mater., 2008, 20, 4116 CrossRef
.
- T. M. Mattox, A. Bergerud, A. Agrawal and D. J. Milliron, Chem. Mater., 2014, 26, 1779 CrossRef CAS
.
- D. W. Lynch, R. Rosei, J. H. Weaver and C. G. Olson, J. Solid State Chem., 1973, 8, 242 CrossRef CAS
.
- I. M. Szilágyi, J. Madarász, G. Pokol, F. Hange, G. Szalontai, K. V. Josepovits and A. L. Tóth, J. Therm. Anal. Calorim., 2009, 97, 11 CrossRef
.
- I. M. Szilágyi, I. Sajó, P. Király, G. Tárkányi, A. L. Tóth, A. Szabo, K. V. Josepovits, J. Madarász and G. Pokol, J. Therm. Anal. Calorim., 2009, 98, 707 CrossRef
.
- C. S. Guo, S. Yin, Y. Huang, Q. Dong and T. Sato, Langmuir, 2011, 27, 12172 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: XRD patterns, zeta potentials, TEM images of the samples, optical absorbance of the powder. See DOI: 10.1039/c4ra12471e |
| This journal is © The Royal Society of Chemistry 2015 |



