A new approach for fused isoindolines via hexadehydro-Diels–Alder reaction (HDDA) by Fe(0) catalysis†
Abstract
A simple method has been developed for the synthesis of fused isoindolines via a cascade HDDA approach catalyzed by Fe2(CO)9. In this work, a 1,3-diyne was involved in a [4 + 2] cycloisomerization with a diynophile to give an aryne intermediate, which was subsequently trapped with –OH nucleophile to beget the fused isoindolines in high yields.
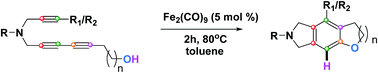

 Please wait while we load your content...
Please wait while we load your content...