Construction and properties of cobalt(II)/copper(II) coordination polymers based on N-donor ligands and polycarboxylates mixed ligands
Hong-Yan Lin,
Jian Luan,
Xiu-Li Wang*,
Ju-Wen Zhang,
Guo-Cheng Liu and
Ai-Xiang Tian
Department of Chemistry, Bohai University, Jinzhou, 121000, P. R. China. E-mail: wangxiuli@bhu.edu.cn; Fax: +86-416-3400158; Tel: +86-416-3400158
First published on 13th November 2014
Abstract
Metal–organic coordination polymers (MOCPs) are well known organic–inorganic hybrids with infinite structures consisting of metal ions/clusters and organic ligands linked through coordination interactions. MOCPs can be constructed from one or more than one organic bridging ligands (mixed-ligands) and different metal ions. The previous reports prove the fact that the nature of organic ligands and metal ions dominates the final structures as well as properties of the MOCPs in a certain way. Therefore, we focus on discussing the cobalt(II)/copper(II) coordination polymers constructed from the mixed-ligands of polycarboxylates and N-donor ligands, which may possess potential applications in the fields of electrochemistry, electrocatalysis, magnetism and photocatalysis. In this review, we summarize some typical Co(II)/Cu(II) MOCPs based on the mixed bridging organic ligands, aiming to discuss their versatile synthesis methods, topologies and structural influence factors, as well as their tunable properties. All of these aspects are highlighted in this review, which seeks to guide further investigations of cobalt(II)/copper(II) coordination polymers.
1 Introduction
Metal–organic coordination polymers (MOCPs) are an intriguing class of hybrid materials, which exist as infinite crystalline lattices extended from inorganic metal ions (or clusters) and organic ligands supported by coordination interactions.1–5 In the past few decades, a variety of typical examples for such crystalline materials with attractive architectures and potential applications in luminescence, heterogeneous catalysis, gas storage/separation, as well as magnetism have attracted significant interest.6–9 The MOCPs containing cobalt(II)/copper(II) ions are particularly interesting because of their excellent physical and chemical properties, especially for their potential applications as electrochemical, electrocatalysis, magnetism and photocatalytic materials.10–17 It is well known that many factors can affect the ultimate structures of the target cobalt(II)/copper(II) MOCPs, such as organic ligands, reactants ratios, systematic pH values and reaction temperatures etc., however, the final structures and properties of cobalt(II)/copper(II) MOCPs are determined in large measure by the nature of organic ligands.10 So the selection of proper organic ligands is an important factor in the self-assembling process of cobalt(II)/copper(II) MOCPs, even small structural changes of the organic ligands such as the symmetry, length, and flexibility or functional groups can produce obvious differences in the final coordination frameworks and topological networks.In recent years, the mixed-ligand system containing two or more organic bridging ligands has been widely adopted to generate new cobalt(II)/copper(II) MOCPs with diversified topologies and interesting properties.11–16 Firstly, the polycarboxylate ligands as a kind of O-donor anionic bridging ligands have been extensively used to construct novel cobalt(II)/copper(II) MOCPs owing to their versatile coordination modes and high structural stability.13–17 Secondly, the neutral N-donor organic ligands can be properly selected as the co-ligands and introduced into the cobalt(II)/copper(II)-polycarboxylate systems, which may connect the cobalt(II)/copper(II) ions/clusters to modify the final architectures and physical properties of target cobalt(II)/copper(II) MOCPs. Thus, this is one of the most efficient synthetic strategies to construct multifunctional MOCPs.
To date, in the reported cobalt(II)/copper(II) MOCPs composed of mixed organic ligands, the neutral multidentate N-donor organic bridging ligands with two or more N and/or O-involving functional groups have been widely applied as the effective tectons, which show different binding abilities and can construct diverse coordination networks.11,12 However, the extensive researches towards the rational design and construction of mixed-ligand cobalt(II)/copper(II) coordination polymers have shown that bis(imidazole)/bis(triazole)/bis(pyridyl) derivatives and polycarboxylate ligands represent the most reliable and typical building blocks which can be jointly applied to synthesize a wide range of desired coordination networks.13–16 A choice of such connectors in coordination assembly can be rationalized based on the following considerations: (i) the neutral N-donor ligands normally bind to the cobalt(II)/copper(II) ions as the rod-like bidentate tectons; (ii) the polycarboxylate ligands can not only provide various coordination modes upon binding to metals, but also play the role of counterions.13–16 As a result, by combining the advantages of such two types of ligands, the so-called mixed-ligand synthetic strategy can be rationally proposed.18,19 Recently, this strategy has also been applied to design and prepare a series of cobalt(II)/copper(II)-based MOCPs with diverse bis(imidazole)/bis(triazole)/bis(pyridyl) and polycarboxylate ligands, which show emerging applications in electrochemistry, magnetism and catalysis.13–16,20–28
This paper would focus on the significant advances in cobalt(II)/copper(II) MOCPs based on N-donor ligands and polycarboxylates mixed-ligands with potential applications in electrochemistry, electrocatalysis, magnetism, and photocatalysis. Part of the ligands discussed in this paper are shown in Schemes 1 and 2.
2 Synthesis method and structural influence factors of the cobalt(II)/copper(II) MOCPs based on the mixed ligands
The acid–base system is the most important and flourishing branch for preparing the cobalt(II)/copper(II) MOCPs based on the mixed ligands.4 Naturally, polycarboxylate anions and cobalt(II)/copper(II) ions are perfect partners that can compensate charge balance, coordination deficiency, and weak interaction all at once.4 In this context, it is valuable to propose the rational synthetic strategy to regulate the networks of cobalt(II)/copper(II) mixed-ligand MOCPs by designing or selecting the suitable neutral N-donor ligands and polycarboxylates, because the intrinsic characteristic of organic ligands (such as spacer, positional isomer and substituent groups) have different effects on the assembly of MOCPs.2,4,29 From another aspect, there are many synthetic methods for obtaining MOCPs in the literatures, such as hydro(solvo)thermal methods,30,31 saturation methods,32 diffusion methods,33 microwave,34 and ultrasonic methods.35 Thereinto, the hydro(solvo)thermal method is originally and commonly used for the synthesis of cobalt(II)/copper(II) MOCPs constructed from the mixed ligands.36–39 Thus, in this paper, cobalt(II)/copper(II) MOCPs containing the mixed ligands synthesized by hydro(solvo)thermal and diffusion method would be focused on.2.1 Cobalt(II)/copper(II) MOCPs based on the mixed ligands by hydrothermal synthesis
Generally, hydrothermal synthesis method can be viewed as the most popular method for preparing cobalt(II)/copper(II) MOCPs based on the mixed ligands.36–64,66–89 Under these conditions, the reduced viscosity of water enhances the diffusion process and thus extraction of solids and crystal growth from solution are favoured.36–64,66–89 During the hydrothermal assembly process, many factors may affect the crystallization and structural construction of the prospective cobalt(II)/copper(II) MOCPs, such as organic ligands (including N-donor ligands and the polycarboxylate ligands), reactants ratio, and pH value, etc. | ||
| Fig. 1 Influence of the polycarboxylic anions on the structures of the MOCPs. Reprinted with permission from ref. 44. | ||
 | ||
| Fig. 2 Influence of the polycarboxylic anions on the structures of the MOCPs. Reprinted with permission from ref. 45. | ||
 | ||
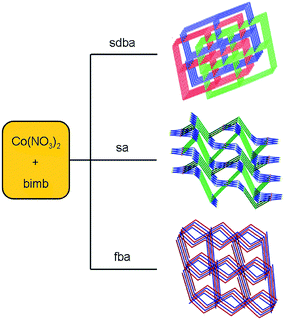
| Fig. 3 Influence of the polycarboxylic anions on the structures of the MOCPs. Reprinted with permission from ref. 46. | ||
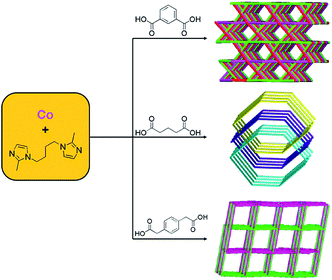
Moreover, the flexible bis(benzimidazole) derivatives as a kind of excellent N-donor ligands show various conformations when coordinating with transition metal ions.54–56 In our previous work, we synthesized a series of coordination polymers based on flexible bis(benzimidazole) and carboxylates mixed ligands (Fig. 4).54–56 In contrast to the flexible bis(benzimidazole) ligands, the semi-rigid bis(benzimidazole) ligands have rigid backbones. In order to explore the effect of the auxiliary ligands on the structures of MOCPs based on the semi-rigid bis(benzimidazole) ligand, different types of carboxylic acids were selected to construct new MOCPs (Fig. 4). As a result, the organic carboxylates have an important effect on the coordination geometries of the Co(II) ions and the final dimensionalities of the complexes.57,58
 | ||
| Fig. 4 Influence of the polycarboxylic anions on the structures of the MOCPs. Reprinted with permission from ref. 57 and 58. | ||
As a part of continuous efforts towards, two pairs of novel complexes have been successfully prepared from the appropriate combination of cobalt(II) ions with the rigid 5-amino-isophthalic acid (aip) (Scheme 2) and flexible bis(imidazole) ligands in different ratios, which further enriches the coordination chemistry of these ligands.48 All of the four complexes possess 2D structures and pack into 3D supramolecular frameworks through intermolecular N–H⋯O hydrogen bonds (Fig. 5). All the frameworks have been analyzed by the topological approach. By comparison of the two pairs of cobalt(II) complexes, when the metal–ligand ratio and flexible linkers are changed, the aip ligand presents various coordinated modes, thus resulting in four unique structures.48
 | ||
| Fig. 5 Influence of the reaction radio on the structures of the MOCPs. Reprinted with permission from ref. 48. | ||
Furthermore, altering bidentate N-donor ligand is an effective strategy for construction of MOCPs with interesting structures and properties.49,50 When using bis-imidazole/bis-pyridyl ligands, four novel cobalt(II) MOCPs based on a flexible 3,3′,4,4′-oxydiphthalic acid (oa) (Scheme 2) have been synthesized through hydrothermal reactions (Fig. 6).49 Moreover, several factors influence the self-assembly process of MOCPs based on flexible organic ligands; among those, the angle between the positions of coordinating groups, i.e. the coordination angle of the organic ligands, is an important factor that alters the dimensionality and topology of the coordination networks.50 The self-assembly of a (flexible, flexible) mixed-ligand system reveals the amendment of dimensionality according to the length of organic ligands, which is dependent on the coordination angle of the organic ligands.50 This concept has been discussed elaborately in some articles by using flexible ligands to prepare a series of complexes.50
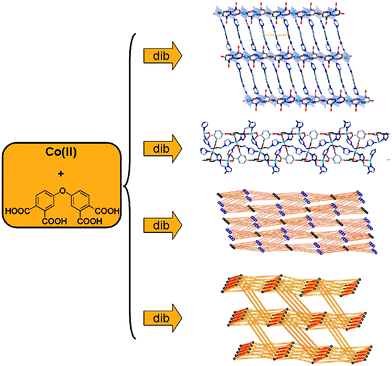 | ||
| Fig. 6 Influence of the N-donor ligands on the structures of the MOCPs. Reprinted with permission from ref. 49. | ||
It is well known that the pH of reaction system plays a crucial role in the construction of metal carboxylate frameworks.51 For 5-nitro-1,2,3-benzenetricarboxylic acid (3-nbtc) (Scheme 2), the pH value of the reaction solution can lead to different degree of deprotonation, which may affect not only the ligand coordination ability but also its charge and even coordination modes.51 In this context, two new complexes assembled by Co(II) salt, 3-nbta and 1,6-bis(triazole)hexane (bth) (Scheme 1) have been synthesized and characterized. The results indicate that pH value of reaction system of metal–H3nbta has vital influence on structures of the result products (Fig. 7).51 Moreover, the steric hindrance created by the secondary ligand at the metal coordination sphere plays an important role in driving the self-assembly process.52,53 By using flexible pyridyl ligands as secondary ligands along with carboxylic acids, the conformation, and position of the ligating atom in the secondary ligand have a substantial role in the formation of diverse architectures.52,53
 | ||
| Fig. 7 Influence of the pH on the structures of the MOCPs. Reprinted with permission from ref. 51. | ||
 | ||
| Fig. 8 (a) Schematic topology for 86 nets; (b) channels in the 3D framework. Reprinted with permission from ref. 59. | ||
As a continuation of such work, six novel copper(II) MOCPs with different topologies have been further synthesized by Ma's group due to the effect of the organic anions.60,61
Recently, Ma's group selected six structurally different organic acids as the anion ligands and two flexible bis(imidazole) ligands as the secondary ligands and synthetized nine new copper(II) MOCPs with different framework structures, and they have investigated the effect of the organic acids on the structures.62 The structural features of the organic acids such as shape, flexibility and length of the spacers are the main reason for the structural differences of the Cu(II) complexes.62 To study the influence of the spacers between the triazole groups on the structures, a copper(II) MOCP was synthesized by using 1,3-bis(1,2,4-triazol-1-ylmethyl)benzene (mbtz) and 1,2,4,5-benzenetetracarboxylate (btec) (Schemes 1 and 2).63 Each btec ligand connects four Cu(II) atoms through its four –COO groups, resulting in a planar 2D [Cu(btec)0.5]n network. The Cu(II) atoms are further coordinated with mbtz ligands to fulfil their coordination geometry. However the mbtz ligands do not increase the dimension of the 2D network.63 In addition, Lysenko and co-workers have employed tetrafunctional adamantanecarboxylic acid (H4adtc) in combination with bistriazole ligands (tr2pr = 1,3-bis(1,2,4-triazol-4-yl)propane and tr2ad = 1,3-bis(1,2,4-triazol-4-yl)adamantane) for the construction of novel Cu(II) MOCPs based on discrete coordination clusters (Fig. 9).64
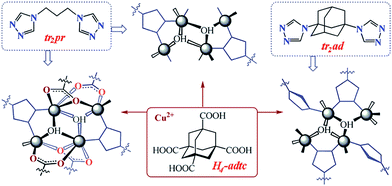 | ||
| Fig. 9 Influence of the N-donor ligands on the structures of the Cu(II) MOCPs. Reprinted with permission from ref. 64. | ||
The triazole and amide groups may provide more potential coordination sites,64–69 and the amide group is an excellent hydrogen acceptor and donor,66–69 which conduces to the formation of hydrogen bonding interactions and may further influences the final structures of target MOCPs. These features may allow bis-triazole-bis-amide ligands, combining the triazole and amide groups, to adopt versatile coordination modes and play an important role in constructing MOCPs.66–69 In order to investigate the effect of polycarboxylates on the networks based on bis-triazole-bis-amide ligands, we designed two flexible bis-triazole-bis-amide ligands with different spacer length to assemble with different aromatic dicarboxylic/tricarboxylic acids, and obtained a series of Cu(II) complexes (Fig. 10).67,69 As a continuation of this kind of ligand, we also designed a semi-rigid bis-triazole-bis-amide N,N′-di(4H-1,2,4-triazole)cyclohexane-1,4-dicarboxamide to construct new MOCPs.66,68
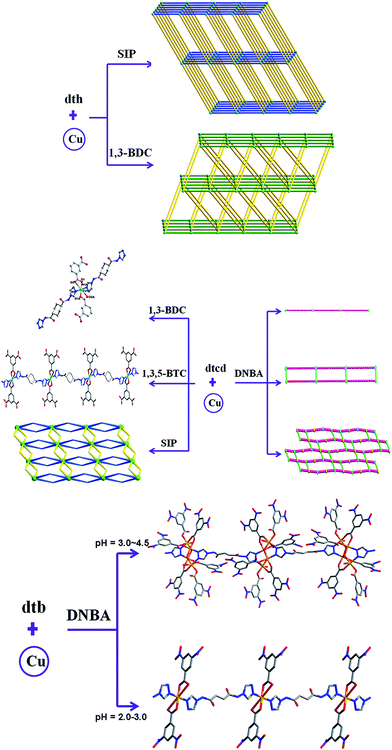 | ||
| Fig. 10 Influence of the polycarboxylic anions, N-donor ligands and pH on the structures of the MOCPs. Reprinted with permission from ref. 66–69. | ||
Cui's group has devoted to exploratory research toward developing new examples of 3D high-connected MOCPs with the mixed nodes based on 1,2,4,5-benzenetetracarboxylic acid (H4btec) and btx (1,4-bis(1,2,4-triazol-1-ylmethyl)-benzene) ligands,70,71 they have successfully synthesized and structurally characterized two new complexes {[Co5(btec)2(btx)(μ3-OH)2(H2O)2]·2H2O}n and [Cu2(btec)(btx)1.5]n, which represent two unusual 3D binodal (4,10)- and (4,7)-connected MOCPs (Fig. 11).
 | ||
| Fig. 11 Influence of the metal ions on the structures of the MOCPs. Reprinted with permission from ref. 70 and 71. | ||
As the contribution of hydrothermal synthesis, our group has been working on the syntheses of Co(II)/Cu(II)-based complexes containing rigid/semi-rigid/flexible bis-pyridyl-bis-amide ligands, and a series of MOCPs with various structures have been constructed.72–89 The structural diversities indicate that the bis-pyridyl-bis-amide ligands not only are good candidates for the construction of coordination polymers but also play an important role in tuning the structures. Moreover, the polycarboxylates also greatly contribute to the structural diversities of the final frameworks.72–89
2.2 Cobalt(II)/copper(II) MOCPs based on the mixed ligands by solvothermal synthesis
The difference of solubility between organic and inorganic components in the same solvent is often a barrier in the formation of single crystals, solvothermal experiments can be a good alternative as solubilities of starting materials can be increased.90–95 This crystallization technique is a non-equilibrium synthesis and may lead to metastable products. This can be influenced mainly by the cooling speed at the end of the reaction.90–95 | ||
| Fig. 12 Influence of the solvents on the structures of the MOCPs. Reprinted with permission from ref. 90. | ||
By using solvothermal synthesis method, three entanglement systems based on the flexible and the rigid bis(imidazole) ligands, including two polyrotaxane-like and one interpenetrating frameworks have been obtained.91 The flexible nature of spacer for the bis(imidazole) allows the ligands to bend and rotate when it coordinates to metal centers. In particular, they favor forming a 2-membered metallacycle when adopting cis-conformation. While the rigid bis(imidazole) ligand tends to construct the coordination polymers exhibiting high porosity, the potential void allows a number of individual nets participating in interpenetration with each other (Fig. 13).91
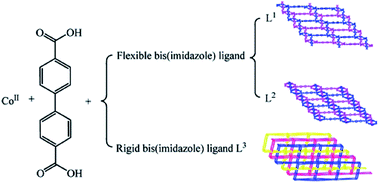 | ||
| Fig. 13 Influence of the N-donor ligands on the structures of the MOCPs. Reprinted with permission from ref. 91. | ||
To investigate the role of three isomeric ligands 1,2-, 1,3-, 1,4-bis(1,2,4-triazol-1-ylmethyl)benzene (1,2-mbtz, 1,3-mbtz, 1,4-mbtz) and rigid 5-nitroisophthalate (nbpdc) (Schemes 1 and 2) in the construction of cobalt(II) MOCPs, three Co(II) MOCPs were synthesized. The structural versatility of the three complexes show that the structures can be tuned by the position isomers. The successful synthesis of these complexes not only provides an intriguing example of a polythreaded system but also provides new perspectives to devise novel extended entanglements (Fig. 14).92
 | ||
| Fig. 14 Influence of the position of N-donor ligands on the structures of the MOCPs. Reprinted with permission from ref. 92. | ||
 | ||
| Fig. 15 Influence of the N-donor ligands on the structures of the MOCPs. Reprinted with permission from ref. 93. | ||
It has been demonstrated that the same Cu(II)/oxalate/twisted N,N′-ligands system can give rise to different complexes with different structures and properties.95 The different complexes are obtained by controlling the synthetic procedures. In this way, four new complexes ranging from a zwitterionic complex to 1D, 2D or 3D MOCPs have been synthesized and characterized. This approach represents an example of the versatility of coordination polymer chemistry (Fig. 16). These complexes have proven to be suitable systems to trap lattice water molecules with different morphologies. In this respect, hydrogen bonds play different, but always crucial, roles in all structures as they can stabilize networks, networks with solvent/guest and solvent/guest with themselves.95
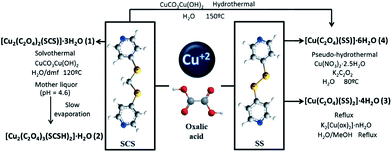 | ||
| Fig. 16 Influence of the pH on the structures of the MOCPs. Reprinted with permission from ref. 95. | ||
2.3 Cobalt(II)/copper(II) MOCPs based on the mixed ligands by diffusion method
Diffusion method is preferred to get single crystals suitable for X-ray diffraction analysis instead of non- or poly-crystalline products, especially if the products are poorly soluble.96,97Of particular interest, the self-assembly of various kinds of coordination frameworks is one of the means to obtain MOCPs with new modes of entanglements in the interpenetrating architectures. Hou et al. have studied the self-assembly of trans, trans-muconic acid (H2muco), a ligand with longer spacer, 4,4′-bis(pyridyl)ethylene (bpe) and cobalt(II) ions by slow diffusion method, then two 3D interpenetrating structures formed in one-pot reaction have been obtained (Fig. 17).96
 | ||
| Fig. 17 Two solvent accessible CPs formed in a one-pot crystallization. Reprinted with permission from ref. 96. | ||
Interestingly, it is easy to get metallogelators from the solvent diffusion method. Dastidar et al. have reported a series of Cu(II)/Co(II) CPs derived from two bis-pyridyl-bis-amide ligands namely N,N′-bis-(3-pyridyl)-isophthalamide (1,3-bpta) and N,N′-bis-(3-pyridyl)terephthalamide (1,4-bpta), and various dicarboxylates (Scheme 3).97
 | ||
| Scheme 3 The outline of the target complexes. Reprinted with permission from ref. 97. | ||
3 The properties and applications of cobalt(II)/copper(II) MOCPs based on the mixed ligands
Cobalt(II)/copper(II) MOCPs have been of great interest in recent years, not only stem from their fascinating structures but also from their potential applications as new materials.98–104 In this part of the review, we will present examples of how the cobalt(II)/copper(II) MOCPs based on the mixed-ligand are promising as materials for applications in the fields of electrochemistry, electrocatalysis, magnetism and photocatalysis.3.1 Electrochemical behaviors and electrocatalytic properties of cobalt(II)/copper(II) CPs based on the mixed ligands
Recently, various efforts have been made to study electrochemistry and electrocatalytic activities of cobalt(II)/copper(II) MOCPs.98–100 Up to now, there are several kinds of method to fabricate chemically modified electrodes (CMEs) according to the way in which materials are attached into the substrates, such as Langmuir–Blodgett (LB),105 chemical vapor deposition (CVD),106 self-assembling (SA),107 drop-casting,108 bulk modification,109 etc. Among of all, chemically bulk-modified carbon paste electrodes (CPE) have been widely used in cobalt(II)/copper(II)-based CPs because of their low background current, easy fabrication and good performance.101,110 Renewing the surface of chemically modified CPE avoids the memory effects and the contamination or deactivation of the surface between consecutive measurements.101,110Recently, our group has been working on the syntheses of Co(II)/Cu(II)-based complexes containing rigid/semi-rigid/flexible bis-triazole/bis-pyridyl ligands, and a series of MOCPs with various electrochemical behaviors have been constructed.54,55,58,67,72–79,83–89
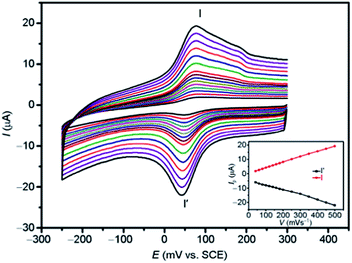 | ||
| Fig. 18 Cyclic voltammograms of the Co-CPE in 0.1 M H2SO4 aqueous solution at different scan rates (from inner to outer: 40, 60, 80, 100, 120, 140, 160, 180, 200, 250, 300, 350, 400, 450 and 500 mV s−1). The inset shows the plots of the anodic and cathodic peak currents against scan rates. Reprinted with permission from ref. 76. | ||
It is well known that the electroreduction of nitrite requires a large overpotential at most electrode surfaces and no obvious response is observed at the bare CPE at the presence of the nitrite. However, for Co-CPE, with the addition of nitrite, the reduction peak currents increase gradually while the corresponding oxidation peak currents decrease dramatically in the range of +300 to −250 mV (Fig. 19). The result indicates that Co-CPE has good electrocatalytic activity toward the reduction of nitrite.
 | ||
| Fig. 19 Cyclic voltammograms of the bare CPE in 0.1 M H2SO4 solution containing 2.0 mmol L−1 KNO2 (a), and Co-CPE in 0.1 M H2SO4 solution containing (b–e): 0.0, 2.0, 4.0 and 6.0 mmol L−1 KNO2. Scan rate: 100 mV s−1. Reprinted with permission from ref. 76. | ||
 | ||
| Fig. 20 Cyclic voltammograms of Cu-CPE in 0.1 M phosphates buffer solution (pH = 2) at different scan rates (from inner to outer) 50, 70, 90, 120, 150, 200, 250, 300, 350 and 400 mV s−1. Inset: plots of peak currents vs. scan rate. Potential vs. SCE. Reprinted with permission from ref. 101. | ||
Bromate is a disinfectant by-product contaminant found in drinking water, and is formed during the ozonation of source water containing bromide.102 The overpotential for BrO3− reduction is high and therefore an efficient electrocatalyst would be beneficial.102 Various types of systems have been reported for bromate detection in the recent years.103 Our work was to fabricate a Cu(II) complex bulk-modified CPE capable of the electrocatalytic reduction of bromate.101 The peak currents for cathodic reduction of bromate are proportional to the bromate concentrations (Fig. 21a).101 These results indicate that at sufficiently negative potential the reaction is controlled by surface-confined of the bromate species, which is the ideal case for quantitative applications.
 | ||
| Fig. 21 Cyclic voltammograms of a bare CPE containing 2 mM KBrO3 (a) KNO2 (b) H2O2 (c) and a Cu-CPE in pH = 2 phosphate buffer solution containing different BrO3− (a) NO2− (b) H2O2 (c) concentrations. Scan rate: 50 mV s−1. Inset: the variation of cathodic peak currents vs. bromate (a) nitrite (b) hydrogen peroxide (c) concentrations. Reprinted with permission from ref. 101. | ||
Some reports on electrocatalytic reduction of nitrite at the surface of MOCPs bulk-modified CPEs have been studied.55,76,85,101 Our work indicates that the Cu(II) complex bulk-modified CPE also shows good electrocatalytic activity toward the reduction of nitrite.101 With addition of nitrite, the reduction peak currents increase markedly while the corresponding oxidation peak currents decrease markedly (Fig. 21b). The electrochemical catalytic pathway is probably the reduction of NO2− to NO and then further reduction to N2O in the aqueous solution.55,76,85,101
H2O2 is usually employed in pollution control, bleaching of textile and paper products and in the processing of petrochemicals, minerals, food and various products.104 Moreover, H2O2 is the product of various oxidases in countless biological reactions, which has been used in the development of first generation biosensors.104 As is known, the electroreduction of H2O2 requires a large overpotential. Fig. 21c proves that the cathodic currents increase while the corresponding anodic currents decrease at the Cu-CPE. The peak currents for cathodic reduction of hydrogen peroxide are proportional to the concentration of H2O2. The process of electroreduction of hydrogen peroxide at the surface of Cu-CPE is similar to that of the bromate and the nitrite.101
3.2 Magnetism properties of cobalt(II)/copper(II) MOCPs based on the mixed ligands
Cobalt(II)/copper(II)-based MOCPs strategy is furthermore employed for the design of molecular-based magnets.2,9,27,111–113 Indeed antiferromagnetism, ferrimagnetism and ferromagnetism are cooperative phenomena of the magnetic spins within a solid. They require an interaction or coupling between the spins of the paramagnetic centres.2,9 The building of metal–organic frameworks allows the choice of the coupling parameters. The magnetic coordination polymers have to own a residual permanent magnetization at zero-field for an as high as possible Tc (critical temperature).2,9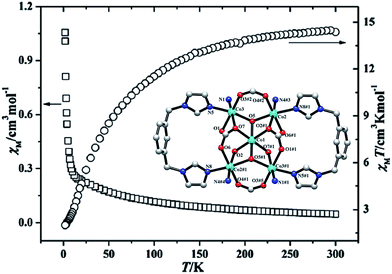 | ||
| Fig. 22 The weak antiferromagnetic interactions between the adjacent Co(II) ions. Reprinted with permission from ref. 111. | ||
As part of efforts in developing new magnets, Bu and co-workers have obtained three new Co(II) MOCPs with a semi-dicarboxylate-like ligands as the subligands.114 The investigation flexible di(1H-imidazol-1-yl)methane ligand and different of the magnetic properties of these complexes demonstrates that they all display FM coupling between Co(II) ions but AFM interaction between Co3 or Co2 units (Fig. 23).
 | ||
| Fig. 23 χmT vs. T plots of the complexes at 1 kOe and 1/χm vs. T plots of the complexes, respectively. The red solid lines are the best fits to the Curie–Weiss laws, respectively. | ||
The magnetic properties of cobalt(II) MOCPs have generally been well explored and they illustrate the potential of producing magnets of many different ferrimagnet,21,24,48,49,114 antiferromagnet21,22,25,38,43,45–57,89,111 and only a few unusual single molecular magnet.26
 | ||
| Fig. 24 (a) The [Cu9(μ3-OH)6] enneanuclear copper(II) cluster; (b) plot of μeff vs. T; (c) Plot of 1/χm vs. T. Reprinted with permission from ref. 112. | ||
 | ||
| Fig. 25 χmT(T) plots. The best fit to eqn is shown as a thin dark line, respectively. Reprinted with permission from ref. 113. | ||
It is clear that copper(II) MOCPs will continue to provide new properties and improve existing knowledge of their structures as well as their magnetic properties. Most of the magnetic properties of copper(II) MOCPs show antiferromagnet67–69,84,9,112 and less of ferrimagnet.27
3.3 Photocatalytic properties of cobalt(II)/copper(II) MOCPs based on the mixed-ligands
Photocatalysis is a “green” technology for the treatment of all kinds of contaminants, which has many advantages over other treatment methods. For instance, the use of the environmentally friendly oxidant O2, which can realize experiments under the ambient temperature reaction condition, and complete the oxidation of the MOCPs, even at low concentrations.115,116 To date, TiO2 has undoubtedly proven to be the most excellent photocatalyst for the oxidative decomposition of many organic pollutants under UV irradiation.117,118 Furthermore, the development of photocatalysis as applied, in particular, to organic pollutants concentration abatement or removal from wastewater under ultraviolet light has received great research attention in catalysis field.115–118 A new emerging application of cobalt(II)/copper(II) MOCPs is photocatalysis, and some MOCPs have been demonstrated to be efficient photocatalysts on the green degradation of organic pollutants.119–121 | ||
| Fig. 26 The UV-vis absorption spectra of the MB (a)/RhB (c)/X3B (e) solution during the decomposition reaction. Photocatalytic degradation of MB (a) RhB (c) X3B (e) solution under UV with the use of the complex; the black curve is the control experiment without any catalyst. Reprinted with permission from ref. 121. | ||
 | ||
| Fig. 27 Concentration changes of X3B as a function of irradiation time for the complex. Conditions: (I) H2O2/complex/dark, (II) complex/visible light, (III) H2O2/visible light, (IV) H2O2/complex/visible light. Reprinted with permission from ref. 122. | ||
4 Conclusions and outlook
The aim of this review is to present a brief summary to introduce the assemblies and structures of cobalt(II)/copper(II)-based mixed-ligand MOCPs with potential applications in electrocatalysis, magnetism, and photocatalytic properties. In this direction, the most effective approach for construction of cobalt(II)/copper(II) MOCPs is to combine both bis-imidazole/bis-triazole/bis-pyridyl derivatives and polycarboxylates by the hydro(solvo)thermal and diffusion methods. Further, the design of organic ligands is mostly practiced by modifying the ligand backbones containing bis-imidazole/bis-triazole/bis-pyridyl functional groups. The mixed-ligand synthetic strategy will be highly affected by the organic ligands (such as the spacers, positional isomer, substituent groups and the types of organic ligands) and some other factors including solvent, pH, metal ion, reactants ratio and synthetic route. These can establish an essential methodology for the rational design and construction of the desired MOCPs with promising properties and potential applications.Although reports on cobalt(II)/copper(II)-based mixed-ligand MOCPs are ever increasing, it is important to recognize that controlling the outcome in terms of the resultant prospective structures and functions is indeed a daunting task. This is because formation of MOCPs is a result of crystallization, which is influenced by numerous and often not controllable factors such as solvent, pH, metal ion, reactants ratio, synthetic route, etc. Generally speaking, the results obtained are unpredictable. Therefore, efforts should be made to understand various factors that influence the formation of the resultant MOCPs. On the other hand, although much of the work during the last decade was motivated by promising properties and potential applications, such as electrocatalysis, magnetism and photocatalysis, several important discoveries in the last few years are opening new and significant opportunities for cobalt(II)/copper(II)-based mixed-ligand MOCPs beyond the gas storage and separations. We foresee that there may be a burgeoning field of porous cobalt(II)/copper(II)-based mixed-ligand MOCPs, which should have a brilliant future and are undergoing explosive growth.
Acknowledgements
The supports of the National Natural Science Foundation of China (no. 21171025, 21471021), New Century Excellent Talents in University (NCET-09-0853) and Program of Innovative Research Team in University of Liaoning Province (LT2012020) are gratefully acknowledged.References
- B. Moulton and M. J. Zaworotko, Chem. Rev., 2001, 101, 1629 CrossRef CAS PubMed.
- A. Y. Robin and K. M. Fromm, Coord. Chem. Rev., 2006, 250, 2127 CrossRef CAS PubMed.
- G. R. Desiraju, Angew. Chem., Int. Ed., 2007, 46, 8342 CrossRef CAS PubMed.
- M. Du, C. P. Li, C. S. Liu and S. M. Fang, Coord. Chem. Rev., 2013, 257, 1282 CrossRef CAS PubMed.
- W. G. Lu, Z. W. Wei, Z. Y. Gu, T. F. Liu, J. Park, J. Park, J. Tian, M. W. Zhang, Q. Zhang, T. Gentle III, M. Boscha and H. C. Zhou, Chem. Soc. Rev., 2014, 43, 5561 RSC.
- M. D. Allendorf, C. A. Bauer, R. K. Bhaktaa and R. J. T. Houka, Chem. Soc. Rev., 2009, 38, 1330 RSC.
- A. Dhakshinamoorthy and H. Garcia, Chem. Soc. Rev., 2012, 41, 5262 RSC.
- J. R. Li, R. J. Kuppler and H. C. Zhou, Chem. Soc. Rev., 2009, 38, 1477 RSC.
- M. Kurmoo, Chem. Soc. Rev., 2009, 38, 1353 RSC.
- O. M. Yaghi, M. O'Keeffe, N. W. Ockwig, H. K. Chae, M. Eddaoudi and J. Kim, Nature, 2003, 423, 705 CrossRef CAS PubMed.
- J. S. Hu, X. Q. Yao, M. D. Zhang, L. Qin, Y. Z. Li, Z. J. Guo, H. G. Zheng and Z. L. Xue, Cryst. Growth Des., 2012, 12, 3426 CAS.
- H. M. He, F. X. Sun, J. T. Jia, Z. Bian, N. Zhao, X. P. Qiu, L. X. Gao and G. S. Zhu, Cryst. Growth Des., 2014, 14, 4258 CAS.
- Z. X. Li, T. L. Hu, H. Ma, Y. F. Zeng, C. J. Li, M. L. Tong and X. H. Bu, Cryst. Growth Des., 2010, 10, 1138 CAS.
- Y. Gong, T. Wu, P. G. Jiang, J. H. Lin and Y. X. Yang, Inorg. Chem., 2013, 52, 777 CrossRef CAS PubMed.
- N. N. Adarsh and P. Dastidar, Chem. Soc. Rev., 2012, 41, 3039 RSC.
- X. Y. Dong, B. Li, B. B. Ma, S. J. Li, M. M. Dong, Y. Y. Zhu, S. Q. Zang, Y. Song, H. W. Hou and T. C. W. Mak, J. Am. Chem. Soc., 2013, 135, 10214 CrossRef CAS PubMed.
- X. Y. Dong, R. Wang, J. B. Li, S. Q. Zang, H. W. Hou and T. C. W. Mak, Chem. Commun., 2013, 49, 10590 RSC.
- M. Kondo, T. Okubo, A. Asami, S. Noro, T. Yoshitomi, S. Kitagawa, T. Ishii, H. Matsuzaka and K. Seki, Angew. Chem., Int. Ed., 1999, 38, 140 CrossRef CAS.
- T. K. Maji, K. Uemura, H. C. Chang, R. Matsuda and S. Kitagawa, Angew. Chem., Int. Ed., 2004, 43, 3269 CrossRef CAS PubMed.
- P. K. Chen, S. R. Batten, Y. Qi and J. M. Zheng, Cryst. Growth Des., 2009, 9, 2756 CAS.
- L. M. Fan, Y. Gao, G. Z. Liu, W. L. Fan, W. K. Song, L. M. Sun, X. Zhao and X. T. Zhang, CrystEngComm, 2014, 16, 7649 RSC.
- L. F. Ma, X. Q. Li, L. Y. Wang and H. W. Hou, CrystEngComm, 2011, 13, 4625 RSC.
- Y. J. Mu, J. H. Fu, Y. J. Song, Z. Li, H. W. Hou and Y. T. Fan, Cryst. Growth Des., 2011, 11, 2183 CAS.
- C. Ren, L. Hou, B. Liu, G. P. Yang, Y. Y. Wang and Q. Z. Shi, Dalton Trans., 2011, 793 RSC.
- N. Wang, Y. C. Feng, W. Shi, B. Zhao, P. Cheng, D. Z. Liao and S. P. Yan, CrystEngComm, 2012, 14, 2769 RSC.
- S. Sanda, S. Parshamoni, A. Adhikary and S. Konar, Cryst. Growth Des., 2013, 13, 5442 CAS.
- J. E. Mizzi and R. L. LaDuca, Inorg. Chim. Acta, 2014, 411, 188 CrossRef CAS PubMed.
- X. L. Wang, B. Mu, H. Y. Lin and G. C. Liu, J. Organomet. Chem., 2011, 696, 2313 CrossRef CAS PubMed.
- X. L. Zhao and W. Y. Sun, CrystEngComm, 2014, 16, 3247 RSC.
- Y. Qi, F. Luo, Y. X. Che and J. M. Zheng, Cryst. Growth Des., 2008, 8, 606 CAS.
- R. Singh and P. K. Bharadwaj, Cryst. Growth Des., 2013, 13, 3722 CAS.
- E. Coronado, J. R. Galán-Mascarós and C. Martí-Gastaldo, CrystEngComm, 2009, 11, 2143 RSC.
- E. Barankova, N. Pradeepa and K. V. Peinemann, Chem. Commun., 2013, 49, 9419 RSC.
- X. F. Wang, Y. B. Zhang, H. Huang, J. P. Zhang and X. M. Chen, Cryst. Growth Des., 2008, 8, 4559 CAS.
- S. Huh, S. Jung, Y. Kim, S. J. Kim and S. Park, Dalton Trans., 2010, 1261 RSC.
- Y. Qi, F. Luo, S. R. Batten, Y. X. Che and J. M. Zheng, Cryst. Growth Des., 2008, 8, 2806 CAS.
- L. P. Zhang, J. F. Ma, J. Yang, Y. Y. Liu and G. H. Wei, Cryst. Growth Des., 2009, 9, 4660 CAS.
- Y. Y. Liu, J. F. Ma, J. Yang and Z. M. Su, Inorg. Chem., 2007, 46, 3027 CrossRef CAS PubMed.
- C. H. Ke, G. R. Lin, B. C. Kuo and H. M. Lee, Cryst. Growth Des., 2012, 12, 3758 CAS.
- B. K. Tripuramallu, P. Manna, S. N. Reddy and S. K. Das, Cryst. Growth Des., 2012, 12, 777 CAS.
- D. Niu, J. Yang, J. Guo, W. Q. Kan, S. Y. Song, P. Du and J. F. Ma, Cryst. Growth Des., 2012, 12, 2397 CAS.
- L. L. Wen, Z. D. Lu, J. G. Lin, Z. F. Tian, H. Z. Zhu and Q. J. Meng, Cryst. Growth Des., 2007, 7, 93 CAS.
- Y. Qi, Y. X. Che, F. Luo, S. R. Batten, Y. Liu and J. M. Zheng, Cryst. Growth Des., 2008, 8, 1654 CAS.
- Z. X. Li, X. Chu, G. H. Cui, Y. Liu, L. Lia and G. L. Xue, CrystEngComm, 2011, 13, 1984 RSC.
- H. Zhou, G. X. Liu, X. F. Wang and Y. Wang, CrystEngComm, 2013, 15, 1377 RSC.
- J. Q. Liu, Y. S. Huang, Y. Y. Zhao and Z. B. Jia, Cryst. Growth Des., 2011, 11, 569 CAS.
- Y. Liu, Y. Qi, Y. H. Su, F. H. Zhao, Y. X. Che and J. M. Zheng, CrystEngComm, 2010, 12, 3283 RSC.
- F. H. Zhao, S. Jing, Y. X. Che and J. M. Zheng, CrystEngComm, 2012, 14, 4478 RSC.
- Q. Chu, Z. Su, J. Fan, T. Okamura, G. C. Lv, G. X. Liu, W. Y. Sun and N. Ueyama, Cryst. Growth Des., 2011, 11, 3885 CAS.
- B. K. Tripuramallu, P. Manna and S. K. Das, CrystEngComm, 2014, 16, 4816 RSC.
- M. L. Han, S. C. Wang and D. F. Feng, Cryst. Res. Technol., 2014, 49, 276 CrossRef CAS.
- P. Manna, B. K. Tripuramallu and S. K. Das, Cryst. Growth Des., 2012, 12, 4607 CAS.
- X. H. Jing, X. C. Yi, E. Q. Gao and V. A. Blatovb, Dalton Trans., 2012, 14316 RSC.
- X. L. Wang, L. L. Hou, J. W. Zhang, J. X. Zhang, G. C. Liu and S. Yang, CrystEngComm, 2011, 13, 3936 Search PubMed.
- X. L. Wang, J. X. Zhang, G. C. Liu, H. Y. Lin, Y. Q. Chen and Z. H. Kang, Inorg. Chim. Acta, 2011, 368, 207 CrossRef CAS PubMed.
- X. L. Wang, J. X. Zhang, L. L. Hou, J. W. Zhang, G. C. Liu and H. Y. Lin, J. Chem. Crystallogr., 2011, 41, 1579 CrossRef CAS PubMed.
- X. L. Wang, L. L. Hou, J. W. Zhang, G. C. Liu and H. Y. Lin, Polyhedron, 2013, 61, 65 CrossRef CAS PubMed.
- L. L. Liu, J. J. Huang, X. L. Wang, G. C. Liu, S. Yang and H. Y. Lin, Inorg. Chim. Acta, 2013, 394, 715 CrossRef CAS PubMed.
- J. F. Ma, J. Yang, G. L. Zheng, S. L. Li and J. F. Liu, Inorg. Chem., 2003, 42, 7531 CrossRef CAS PubMed.
- J. Yang, J. F. Ma, Y. Y. Liu, S. L. Li and G. L. Zheng, Eur. J. Inorg. Chem., 2005, 2005, 2174 CrossRef.
- J. Yang, J. F. Ma, Y. Y. Liu, J. C. Ma, H. Q. Jia and N. H. Hu, Eur. J. Inorg. Chem., 2006, 2006, 1208 CrossRef.
- J. Yang, J. F. Ma, Y. Y. Liu, J. C. Ma and S. R. Batten, Cryst. Growth Des., 2008, 8, 4383 CAS.
- H. Y. Ge, Y. F. Peng, J. G. Ding, B. L. Li and Y. Zhang, Chin. J. Chem., 2012, 30, 1479 CrossRef CAS.
- G. A. Senchyk, A. B. Lysenko, H. Krautscheid, E. B. Rusanov, A. N. Chernega, K. W. Krämer, S. Xia Liu, S. Decurtins and K. V. Domasevitch, Inorg. Chem., 2013, 52, 863 CrossRef CAS PubMed.
- L. Tian, Z. Chen, A. Yu, H. B. Song and P. Cheng, CrystEngComm, 2012, 14, 2032 RSC.
- X. L. Wang, W. Zhao, J. W. Zhang, Q. L. Lu, J. Luan, G. C. Liu, H. Y. Lin and A. X. Tian, J. Coord. Chem., 2013, 66, 3561 CrossRef CAS.
- Q. L. Lu, W. Zhao, J. W. Zhang, X. L. Wang and J. Luan, Z. Anorg. Allg. Chem., 2012, 693, 587 Search PubMed.
- X. L. Wang, W. Zhao, J. L. Hou, J. W. Zhang, Q. L. Lu, J. Luan and G. C. Liu, Transition Met. Chem., 2013, 38, 827 CrossRef CAS.
- J. W. Zhang, W. Zhao, Q. L. Lu, J. Luan, Y. Qu and X. L. Wang, J. Solid State Chem., 2014, 212, 151 CrossRef CAS PubMed.
- J. M. Hao, L. N. Wang, K. V. Hecke and G. H. Cui, Inorg. Chem. Commun., 2014, 41, 43 CrossRef CAS PubMed.
- G. H. Cui, C. H. He, C. H. Jiao, J. C. Geng and V. A. Blatov, CrystEngComm, 2012, 14, 4210 RSC.
- X. L. Wang, H. Y. Lin, B. Mu, A. X. Tian and G. C. Liu, Dalton Trans., 2010, 6187 RSC.
- X. L. Wang, H. Y. Lin, B. Mu, A. X. Tian, G. C. Liu and N. H. Hu, CrystEngComm, 2011, 13, 1990 RSC.
- X. L. Wang, B. Mu, H. Y. Lin, G. C. Liu, A. X. Tian and S. Yang, CrystEngComm, 2012, 14, 1001 RSC.
- X. L. Wang, B. Mu, H. Y. Lin, S. Yang, G. C. Liu, A. X. Tian and J. W. Zhang, Dalton Trans., 2012, 11074 RSC.
- X. L. Wang, B. Mu, H. Y. Lin, S. Yang, G. C. Liu, A. X. Tian and J. W. Zhang, Sci. China: Chem., 2013, 56, 557 CrossRef CAS.
- Q. L. Lu, J. Luan, X. L. Wang, H. Y. Lin and C. Xu, Transition Met. Chem., 2012, 37, 713 CrossRef CAS.
- X. L. Wang, J. Luan, Q. L. Lu, H. Y. Lin and C. Xu, J. Organomet. Chem., 2012, 719, 1 CrossRef CAS PubMed.
- X. L. Wang, J. Luan, H. Y. Lin, Q. L. Lu, C. Xu and G. C. Liu, Dalton Trans., 2013, 8375 RSC.
- X. L. Wang, J. Luan, F. F. Sui, H. Y. Lin, G. C. Liu and C. Xu, Cryst. Growth Des., 2013, 13, 3561 CAS.
- X. L. Wang, J. Luan, H. Y. Lin, C. Xu and G. C. Liu, Inorg. Chim. Acta, 2013, 408, 139 CrossRef CAS PubMed.
- X. L. Wang, J. Luan, H. Y. Lin, C. Xu, G. C. Liu, J. W. Zhang and A. X. Tian, CrystEngComm, 2013, 15, 9995 RSC.
- X. L. Wang, J. Luan, H. Y. Lin, G. C. Liu and M. Le, Polyhedron, 2014, 71, 111 CrossRef CAS PubMed.
- X. L. Wang, P. Liu, H. Y. Lin, C. Xu, J. W. Zhang and G. C. Liu, Inorg. Chem. Commun., 2013, 30, 79 CrossRef CAS PubMed.
- X. L. Wang, J. Luan, H. Y. Lin, M. Le and G. C. Liu, Dalton Trans., 2014, 8072 RSC.
- X. L. Wang, J. Luan, H. Y. Lin, Q. L. Lu, M. Le and G. C. Liu, J. Mol. Struct., 2014, 1074, 441 CrossRef CAS PubMed.
- X. L. Wang, F. F. Sui, H. Y. Lin, J. Luan and G. C. Liu, Aust. J. Chem., 2013, 66, 67 CrossRef CAS.
- X. L. Wang, F. F. Sui, H. Y. Lin, C. Xu, G. C. Liu, J. W. Zhang and A. X. Tian, CrystEngComm, 2013, 15, 7274 RSC.
- X. L. Wang, F. F. Sui, H. Y. Lin, J. W. Zhang and G. C. Liu, Cryst. Growth Des., 2014, 14, 3438 CAS.
- S. Q. Zang, M. M. Dong, Y. J. Fan, H. W. Hou and T. C. W. Mak, Cryst. Growth Des., 2012, 12, 1239 CAS.
- Y. Liu, Y. Qi, Y. Y. Lv, Y. X. Che and J. M. Zheng, Cryst. Growth Des., 2009, 9, 4797 CAS.
- X. Zhu, P. P. Sun, J. G. Ding, B. L. Li and H. Y. Li, Cryst. Growth Des., 2012, 12, 3992 CAS.
- M. M. Dong, L. L. He, Y. J. Fan, S. Q. Zang, H. W. Hou and T. C. W. Mak, Cryst. Growth Des., 2013, 13, 3353 CAS.
- H. Wang, V. Safarifard, S. Y. Wang, L. H. Tu, H. P. Xiao, B. F. Huang, X. H. Li, M. Payehghadrc and A. Morsali, RSC Adv., 2014, 4, 11423 RSC.
- A. B. Lago, R. Carballo, O. Fabelo, N. Fernández-Hermida, F. Lloret and E. M. Vázquez-Lópeza, CrystEngComm, 2013, 15, 10550 RSC.
- M. H. Mir, S. Kitagawa and J. J. Vittal, Inorg. Chem., 2008, 47, 7728 CrossRef CAS PubMed.
- N. N. Adarsh, P. Sahoo and P. Dastidar, Cryst. Growth Des., 2010, 10, 4976 CAS.
- S. Pandey, P. P. Das, A. K. Singh and R. Mukherjee, Dalton Trans., 2011, 10758 RSC.
- A. K. Singh and R. Mukherjee, Dalton Trans., 2005, 2886 RSC.
- S. Hazra, S. Majumder, M. Fleck, N. Aliaga-Alcalde and S. Mohanta, Polyhedron, 2009, 28, 3707 CrossRef CAS PubMed.
- X. L. Wang, H. Y. Zhao, H. Y. Lin, G. C. Liu, J. N. Fang and B. K. Chen, Electroanalysis, 2008, 20, 1055 CrossRef CAS.
- N. Fay, E. Dempsey and T. Mccormac, J. Electroanal. Chem., 2005, 574, 359 CrossRef CAS PubMed.
- A. Salimi, V. Alizadeh and H. Hadadzadeh, Electroanalysis, 2004, 16, 1984 CrossRef CAS.
- G. L. L. Gonzalez, H. Kahlert and F. Scholz, Electrochim. Acta, 2007, 52, 1968 CrossRef PubMed.
- H. M. Dong, L. Lin, H. Zheng, G. X. Zhao and B. X. Ye, Electroanalysis, 2006, 18, 1202 CrossRef CAS.
- J. H. Yu and H. X. Ju, Anal. Chem., 2002, 74, 3579 CrossRef CAS.
- L. H. Xu, Y. H. Zhu, L. H. Tang, X. L. Yang and C. Z. Li, Electroanalysis, 2007, 19, 717 CrossRef CAS.
- W. P. Wuelfing, F. P. Zamborini, A. C. Templeton, X. G. Wen, H. Yoon and R. W. Murray, Chem. Mater., 2000, 13, 87 CrossRef.
- N. Maleki, A. Safavi and F. Tajabadi, Anal. Chem., 2006, 78, 3820 CrossRef CAS PubMed.
- X. L. Wang, E. B. Wang and C. W. Hu, Chem. Lett., 2001, 1030 CrossRef CAS.
- M. L. Han, Y. P. Duan, D. S. Li, H. B. Wang, J. Zhao and Y. Y. Wang, Dalton Trans., 2014, 15450 RSC.
- X. Zhu, S. Zhao, Y. F. Peng, B. L. Li and B. Wu, CrystEngComm, 2013, 15, 9154 RSC.
- S. H. Qiblawi, A. L. Pochodylo and R. L. LaDuca, CrystEngComm, 2013, 15, 8979 RSC.
- Q. Yang, X. F. Zhang, J. P. Zhao, B. W. Hu and X. H. Bu, Cryst. Growth Des., 2011, 11, 2839 CAS.
- A. Fujishima and K. Honda, Nature, 1972, 238, 37 CrossRef CAS.
- S. W. Liu, J. G. Yu and M. Jaroniec, J. Am. Chem. Soc., 2010, 132, 11914 CrossRef CAS PubMed.
- T. Tsumura, N. Kojitan, I. Izumi, N. Iwashita, M. Toyoda and M. Inagaki, J. Mater. Chem., 2002, 12, 1391 RSC.
- M. Ksibi, S. Rossignol, J. M. Tatibouet and C. Trapalis, Mater. Lett., 2008, 62, 4204 CrossRef CAS PubMed.
- S. Zhou, Z. G. Kong, Q. W. Wang and C. B. Li, Inorg. Chem. Commun., 2012, 25, 1 CrossRef CAS PubMed.
- L. L. Wen, F. Wang, J. Feng, K. L. Lv, C. G. Wang and D. F. Li, Cryst. Growth Des., 2009, 9, 3581 CAS.
- J. Guo, J. Yang, Y. Y. Liu and J. F. Ma, CrystEngComm, 2012, 14, 6609 RSC.
- L. L. Wen, J. B. Zhao, K. L. Lv, Y. H. Wu, K. J. Deng, X. K. Leng and D. F. Li, Cryst. Growth Des., 2012, 12, 1603 CAS.
| This journal is © The Royal Society of Chemistry 2014 |


