Influence of solvents and assembly methods on the supramolecular patterns and luminescent properties of organic salts comprising 4,4′-dihydroxybiphenyl-3,3′-disulfonate and triphenylmethanaminium†
Ya-Nan Li,
Li-Hua Huo,
Yi-Zhe Yu,
Fa-Yuan Ge,
Zhao-Peng Deng*,
Zhi-Biao Zhu and
Shan Gao*
Key Laboratory of Functional Inorganic Material Chemistry, Ministry of Education, School of Chemistry and Materials Science, Heilongjiang University, Harbin 150080, People's Republic of China. E-mail: jackdeng2001@yahoo.com; shangao67@yahoo.com; Fax: +86-0451-86608040; Tel: +86-0451-86608426
First published on 19th November 2014
Abstract
Twelve organic salts, namely 2(HTPMA)+·(H2M)2−·4(H2O) (1), 2(HTPMA)+·(H2M)2−·2(H2O) (2), 2(HTPMA)+·(H2M)2−·2(MeOH)·(H2O) (3), 2(HTPMA)+·(H2M)2−·4(MeOH) (4), 2(HTPMA)+·(H2M)2−·(MeOH) (5), 2(HTPMA)+·(H2M)2−·2(EtOH)·2(H2O) (6), 2(HTPMA)+·(H2M)2−·2(n-PrOH) (7), 2(HTPMA)+·(H2M)2−·2(n-BuOH) (8), 2(HTPMA)+·(H2M)2−·2(n-PeOH) (9), 2(HTPMA)+·(H2M)2−·2(DO) (10), 2(HTPMA)+·(H2M)2−·2(DMF) (11), and 2(HTPMA)+·(H2M)2−·2(DMSO) (12) (H4M = 4,4′-dihydroxybiphenyl-3,3′-disulfonic acid, TPMA = triphenylmethylamine, DO = 1,4-dioxane) have been obtained from the reaction of H4M and TPMA in different solvents by two assembly methods and characterized by elemental analysis, IR, TG, PL, powder and single-crystal X-ray diffraction. Structural analyses indicate that the nature of the solvent molecules can effectively influence the ⋯(–SO3)⋯(–NH3)⋯(solvent)⋯ patterns, which then result in diverse packing diagrams. In salts 1 and 3, pairs of HTPMA+ cations arrange in a tail-to-tail mode to form column motifs which extend the layers of H2M2− dianions into a pillared layered network. On the contrary, pairs of HTPMA+ cations in salt 2 arrange in head-to-head mode and form layer structures together with pairs of H2M2− dianions. The HTPMA+ cations and H2M2− dianions in salts 4 and 6 are alternately arranged to form a column motif, which then pack with each other to form a supramolecular network. Pairs of head-to-head HTPMA+ cations in salts 7–9 are sandwiched between the –SO3 groups through hydrogen bonding interactions, generating a graphite-like structure. The HTPMA+ cations in salts 5 and 10–12 arrange in tail-to-tail mode to form column motifs which are then sandwiched between biphenyl rings instead of the –SO3 groups. Moreover, different assembly processes are also responsible for the diverse structures. Small solvent molecules, such as H2O and MeOH, tend to form different structures (1 and 2, 3 and 4), while large molecules usually present the same structures (6–12). It is interesting to note that salt 4 can transform into salt 5 after being exposed to the air for several hours. Luminescence investigation reveals that the emission maximum of salts 1–12 varies from 365 to 371 nm.
Introduction
Supramolecular chemistry and crystal engineering have recently developed rapidly with the ultimate goal of obtaining new solids with designed structures and desired physical and chemical properties (e.g. photochemistry, biomedicine and pharmaceutics) through noncovalent synthesis.1–4 To carry out such an aim, careful selection of synthons would be the primary and crucial issue owing to the fact that the nature of synthons has a large effect on the type and character of the noncovalent interactions, which then influence the architectures and properties of target supramolecules.5 In this sense, the sulfonate anion (–SO3−) and –NH3+ cation are good candidates for their same ‘AB3’ form and C3v symmetry. The three acceptor oxygen atoms on the –SO3− themselves have a closely related tetrahedral geometry to the three donor H atoms on the –NH3+ cations. Thus, the equal number of separated hydrogen bond donor and acceptor sites with matched geometry and stereoavailability allows the formation of high symmetrical supramolecular patterns.6 To date, a series of outstanding works have been reported by Tohnai,7 our8 and other research groups9 by using diverse aliphatic or aromatic sulfonates and ammoniums.Besides, during the assemble process, different external stimuli, especially solvents, are attractive for inducing the potential structural diversities of supramolecular systems and their structurally-related properties.10 Generally, such regulating on structures and properties are achieved by utilizing the solvent-dependent supramolecular interactions (e.g. hydrogen-bonding interactions and hydrophobic interactions) and the nature of the solvent. To date, a few solvent-dependent solid-state structure and luminescence for ammonium sulfonates have been demonstrated by Tohnai and co-workers. In 2006, three isostructural organic salts were obtained from the anthrace-2,6-disulfonic acid and racemic sec-butylamine, which captured the dioxane, thioxane and benzene molecules in their channel-like cavities. The three salts exhibited luminescent emission maximum at 438, 423 and 427 nm, respectively.7a Another intriguing instance was reported in 2011,7e in which they recrystallized the supramolecular complexes comprising triphenylmethylamine (TPMA) and anthrace-1,8-disulfonic acid from mixtures of methanol and a variety of organic solvents. These organic salts are all exhibiting similar structures with the packing of supramolecular beads with the guest solvent molecules filled in the formed channels. Meanwhile, the maximum wavelength of the fluorescence emission spectra (λem) widely shifted from 452 to 570 nm. The guest molecules can be adsorbed into a channel-like cavity, and the solid-state fluorescence of the organic salts change according to the presence of guest molecules. Moreover, from the viewpoint of synthetic chemistry, assemble process is also an important factor to modulate the architectures and properties of target supramolecules. Up to now, some prominent instances incorporating metal–organic complexes have been reported.11 In contrast, the influence of different assemble process in the construction of organic salts, especially ammonium sulfonates, is rarely concerned.
Bear the above information in mind, we designed a new organic molecule, 4,4′-dihydroxybiphenyl-3,3′-disulfonic acid (H4M, Scheme 1), based on the following points: (i) two sulfonate groups (–SO3−), with two types of conformations, can form intensive hydrogen bonding interactions and subsequently diverse hydrogen-bonding patterns with various donors, such as the primary ammonium cations (R–NH3+) and different solvent molecules. (ii) The two hydroxyl groups act as both donor and acceptor to involve in the formation of hydrogen bonding interactions, thus reducing the C3v symmetry of –SO3− and further modulating the supramolecular network of target molecules. (iii) The longer spacer of biphenyl ring could afford the formation of large hydrophobic cavity with large primary amine, such as TPMA, to capture the solvent molecules. Meanwhile, the emanative style of the three phenyl rings of TPMA is responsible for the arrangement of different solvent molecules based on their different character, which then lead to the variation of architectures and luminescent emissions. According to these considerations and solubility of reactants, a variety of solvents with different volume and ability for building hydrogen bonds are selected to study their effects on the supramolecular patterns and luminescent properties of organic salts comprising H4M and TPMA under different assemble processes. Subsequently, we reported here the supramolecular patterns and luminescent properties of twelve salts assembled by H4M and TPMA in different solvents, namely, 2(HTPMA)+·(H2M)2−·4(H2O) (1), 2(HTPMA)+·(H2M)2−·2(H2O) (2), 2(HTPMA)+·(H2M)2−·2(MeOH)·(H2O) (3), 2(HTPMA)+·(H2M)2−·4(MeOH) (4), 2(HTPMA)+·(H2M)2−·(MeOH) (5), 2(HTPMA)+·(H2M)2−·2(EtOH)·2(H2O) (6), 2(HTPMA)+·(H2M)2−·2(n-PrOH) (7), 2(HTPMA)+·(H2M)2−·2(n-BuOH) (8), 2(HTPMA)+·(H2M)2−·2(n-PeOH) (9), 2(HTPMA)+·(H2M)2−·2(DO) (10), 2(HTPMA)+·(H2M)2−·2(DMF) (11), and 2(HTPMA)+·(H2M)2−·2(DMSO) (12) (TPMA = triphenylmethylamine, DO = 1,4-dioxane). It can be concluded from the structural analyses that the nature of the solvent molecules and different assemble processes can effectively influence the hydrogen bonding modes of the –SO3 and –NH3 groups, which then result in diverse architectures. Interestingly, after being exposed to the air for several hours, salt 5 could be obtained from the transformation of salt 4. Thermal stabilities and luminescent properties of the twelve salts are also investigated.
Experimental
Materials and methods
H4M was synthesized and purified according to our previous reported method.12 Other chemicals and solvents were of A. R. grade and used without further purification. Elemental analyses were carried out with a Vario MICRO from Elementar Analysensysteme GmbH, and the infrared spectra (IR) of KBr pellets were recorded in the range of 4000–400 cm−1 on a Bruker Equinox 55 FT-IR spectrometer. Powder X-ray diffraction (PXRD) patterns were collected at 293 K on a Bruker D8 diffractometer (Cu Kα, λ = 1.54059 Å). The TG analyses were carried out on a Perkin-Elmer TG/DTA 6300 thermal analyzer under flowing N2 atmosphere, with a heating rate of 10 °C min−1. Luminescence spectra were measured on a Perkin-Elmer LS 55 luminescence spectrometer.Preparation of complexes
Salt 1. Yield: 84% (based on S). Elemental analysis calcd (%) for C50H52N2O12S2: C 64.09, H 5.59, N 2.99; found: C 64.12, H 5.65, N 2.97. Main IR (ν/cm−1): 3432br,s, 3173m, 3067–2907br,s, 1612s, 1548m, 1484s, 1448m, 1384m, 1244s, 1219s, 1166s, 1088s, 1016s.
Salt 3. Yield: 80% (based on S). Elemental analysis calcd (%) for C52H54N2O11S2: C 65.94, H 5.75, N 2.96; found: C 65.90, H 5.81, N 2.98. Main IR (ν/cm−1): 3421br,s, 3256m, 3062–2848br,s, 1612s, 1539m, 1482s, 1449m, 1384m, 1228s, 1207s, 1159s, 1089s, 1020s.
Salt 6. Yield: 76% (based on S). Elemental analysis calcd (%) for C54H60N2O12S2: C 65.30, H 6.09, N 2.82; found: C 65.33, H 6.02, N 2.85. Main IR (ν/cm−1): 3406s, 3251m, 3060–2873br,s, 1614s, 1539m, 1475s, 1446m, 1381m, 1257s, 1203s, 1157s, 1085s, 1016s.
Salt 7. Yield: 78% (based on S). Elemental analysis calcd (%) for C56H60N2O10S2: C 68.27, H 6.14, N 2.84; found: C 68.23, H 6.22, N 2.87. Main IR (ν/cm−1): 3359s, 3259m, 3058–2860br,s, 1608s, 1538m, 1473s, 1448s, 1378m, 1249s, 1212s, 1166s, 1082s, 1020s.
Salt 8. Yield: 82% (based on S). Elemental analysis calcd (%) for C58H64N2O10S2: C 68.75, H 6.37, N 2.76; found: C 68.78, H 6.31, N 2.74. Main IR (ν/cm−1): 3374m, 3272m, 3051–2868br,s, 1612s, 1541m, 1473s, 1448m, 1380m, 1257s, 1210s, 1160s, 1088s, 1021s.
Salt 9. Yield: 79% (based on S). Elemental analysis calcd (%) for C60H68N2O10S2: C 69.21, H 6.58, N 2.69; found: C 69.24, H 6.53, N 2.66. Main IR (ν/cm−1): 3384m, 3268m, 3054–2859br,s, 1610s, 1541m, 1473s, 1448m, 1378m, 1249s, 1213s, 1164s, 1087s, 1020s.
Salt 10. Yield: 84% (based on S). Elemental analysis calcd (%) for C58H60N2O12S2: C 66.90, H 5.81, N 2.69; found: C 66.86, H 5.84, N 2.71. Main IR (ν/cm−1): 3229m, 3068–2861br,s, 1612s, 1532m, 1475s, 1448m, 1382m, 1257s, 1218s, 1153s, 1085s, 1016s.
Salt 11. Yield: 85% (based on S). Elemental analysis calcd (%) for C56H58N4O10S2: C 66.52, H 5.78, N 5.54; found: C 66.54, H 5.81, N 5.52. Main IR (ν/cm−1): 3276m, 3037–2864br,s, 1662s, 1612s, 1542m, 1473s, 1448m, 1378m, 1257s, 1214s, 1155s, 1089s, 1013m.
Salt 12. Yield: 82% (based on S). Elemental analysis calcd (%) for C54H56N2O10S4: C 63.51, H 5.53, N 2.74; found: C 63.47, H 5.58, N 2.71. Main IR (ν/cm−1): 3236m, 3091–2899br,s, 1608s, 1538m, 1480s, 1449m, 1384m, 1251s, 1226s, 1191s, 1095s, 1014s.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio was well mixed and grinded for 1.5 hours without any solvent. Then, the grinding mixture was separately dissolved in the aforementioned solvents and evaporated at room temperature. After several days, the same crystals of salts 6–12 in ethanol, n-propanol, n-butanol, n-pentanol, 1,4-dioxane, dimethylformamide and dimethyl sulfoxide were obtained while another two new salts (2 and 4) were obtained from water and methanol.
2 ratio was well mixed and grinded for 1.5 hours without any solvent. Then, the grinding mixture was separately dissolved in the aforementioned solvents and evaporated at room temperature. After several days, the same crystals of salts 6–12 in ethanol, n-propanol, n-butanol, n-pentanol, 1,4-dioxane, dimethylformamide and dimethyl sulfoxide were obtained while another two new salts (2 and 4) were obtained from water and methanol.
Salt 2. Yield: 76% (based on S). Elemental analysis calcd (%) for C50H50N2O11S2: C 65.34, H 5.48, N 3.05; found: C 65.36, H 5.43, N 3.08. Main IR (ν/cm−1): 3452m, 3274m, 3067–2974br,s, 1613s, 1546m, 1477s, 1446m, 1379m, 1238s, 1221s, 1159s, 1087s, 1014s.
Salt 4. Yield: 84% (based on S). Elemental analysis calcd (%) for C54H60N2O12S2: C 65.30, H 6.09, N 2.82; found: C 65.34, H 6.04, N 2.84. Main IR (ν/cm−1): 3396m, 3255m, 3060–2853br,s, 1612s, 1539m, 1477s, 1446m, 1379m, 1249s, 1203s, 1159s, 1088s, 1016s.
X-ray crystallographic measurements
Table 1 provides a summary of the crystal data, data collection and refinement parameters for 2(H3O)+·(H2M)2− and salts 1–12. All diffraction data were collected at 295 K on a Xcalibur Eos diffractometer with graphite monochromatized Mo-Kα (λ = 0.71073 Å) radiation in ω scan mode. All structures were solved by direct method and difference Fourier syntheses. All non-hydrogen atoms were refined by full-matrix least-squares techniques on F2 with anisotropic thermal parameters. The hydrogen atoms attached to carbons in these complexes and hydroxyl oxygens (O5) in salts 6 and 8 were placed in calculated positions with C–H = 0.93 Å (aromatic H atoms), C–H = 0.97 Å (methylene H atoms), C–H = 0.96 Å (methyl H atoms), O–H = 0.82 Å, and U (H) = 1.2Ueq (C) and 1.5Ueq (O) in the riding model approximation. The hydrogen atoms attached to nitrogens, other hydroxyl oxygens and oxygens of water molecules were located in difference Fourier maps and were also refined in the riding model approximation, with N–H and O–H distance restraint (0.86(1) or 0.85(1) Å) and U(H) = 1.5Ueq (N, O). The O2w in salt 2, O1w in salt 6, as well as the n-propanol, n-butanol, n-pentanol molecules in salts 7–9 are all disordered over two positions with the ratio of 0.50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.50. The sulfonate groups in salts 7–9 are disordered over two positions with the ratio of 0.66
0.50. The sulfonate groups in salts 7–9 are disordered over two positions with the ratio of 0.66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.34, 0.61
0.34, 0.61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.39 and 0.53
0.39 and 0.53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.47, respectively. All calculations were carried out with the SHELXTL97 program.13 The CCDC reference numbers are1003905 and 1025993–1026004 for 2(H3O)+·(H2M)2− and salts 1–12. Selected hydrogen bond parameters for 2(H3O)+·(H2M)2− and salts 1–12 are presented in Table 2.
0.47, respectively. All calculations were carried out with the SHELXTL97 program.13 The CCDC reference numbers are1003905 and 1025993–1026004 for 2(H3O)+·(H2M)2− and salts 1–12. Selected hydrogen bond parameters for 2(H3O)+·(H2M)2− and salts 1–12 are presented in Table 2.
| Salts | 2(H3O)+·(H2M)2− | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|
| Empirical formula | C12H14O10S2 | C50H52N2O12S2 | C50H50N2O11S2 | C52H54N2O11S2 | C54H60N2O12S2 |
| Formula weight | 382.35 | 937.06 | 919.04 | 947.09 | 993.16 |
| Space group | C2/c | P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
C2/c | P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
| a/Å | 29.188(7) | 12.9215(9) | 27.0976(12) | 13.0823(9) | 10.0429(6) |
| b/Å | 5.7170(10) | 13.6231(8) | 12.9962(4) | 13.6545(8) | 12.1542(7) |
| c/Å | 22.742(8) | 14.7007(12) | 13.9750(8) | 14.8789(7) | 12.5456(8) |
| α/° | 90.00 | 93.768(6) | 90.00 | 98.884(5) | 67.646(6) |
| β/° | 120.89 | 101.133(7) | 103.679(5) | 94.355(5) | 67.742(6) |
| γ/° | 90.00 | 110.031(6) | 90.00 | 110.469(6) | 80.119(5) |
| V/Å−3 | 3256.6(15) | 2361.0(3) | 4781.9(4) | 2435.8(2) | 1310.07(13) |
| Z | 8 | 2 | 4 | 2 | 1 |
| Dc/g cm−3 | 1.560 | 1.318 | 1.277 | 1.291 | 1.259 |
| μ (Mo Kα)/mm−1 | 0.377 | 0.178 | 0.173 | 0.172 | 0.164 |
| F(000) | 1584 | 988 | 1936 | 1000 | 526 |
| Reflections collected | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 272 272 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 038 038 |
8515 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 501 501 |
7751 |
| Unique reflections | 3725 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 745 745 |
5476 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 090 090 |
5968 |
| Parameters | 241 | 643 | 313 | 642 | 336 |
| R (int) | 0.0567 | 0.0188 | 0.0214 | 0.0180 | 0.0229 |
| GOF on F2 | 1.062 | 1.029 | 1.027 | 1.025 | 1.022 |
| Final R indices [I ≥ 2σ(I)] | R1= 0.0521, wR2 = 0.1352 | R1 = 0.0576, wR2 = 0.1280 | R1 = 0.0660, wR2 = 0.1652 | R1 = 0.0573, wR2 = 0.1208 | R1 = 0.0580, wR2 = 0.1308 |
| Salts | 5 | 6 | 7 | 8 |
|---|---|---|---|---|
| Empirical formula | C51H48N2O9S2 | C54H60N2O12S2 | C50H60N2O10S2 | C58H64N2O10S2 |
| Formula weight | 897.03 | 993.16 | 985.18 | 1013.23 |
| Space group | P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
C2/c | C2 |
| a/Å | 12.9200(7) | 9.6061(19) | 25.2368(15) | 25.3691(12) |
| b/Å | 13.9363(6) | 12.081(2) | 7.9675(4) | 8.0242(5) |
| c/Å | 13.9627(6) | 12.588(3) | 28.3845(16) | 15.2135(7) |
| α/° | 87.493(4) | 68.92(3) | 90.00 | 90.00 |
| β/° | 85.351(4) | 72.30(3) | 111.958(7) | 119.171(3) |
| γ/° | 63.093(5) | 82.30(3) | 90.00 | 90.00 |
| V/Å−3 | 2234.47(18) | 1298.1(4) | 5293.4(5) | 2704.2(2) |
| Z | 2 | 1 | 4 | 2 |
| Dc/g m−3 | 1.333 | 1.270 | 1.236 | 1.244 |
| μ (Mo Kα)/mm−1 | 0.180 | 0.166 | 0.159 | 0.158 |
| F(000) | 944 | 526 | 2088 | 1076 |
| Reflections collected | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 260 260 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 052 052 |
9921 | 5270 |
| Unique reflections | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 178 178 |
4514 | 6065 | 4702 |
| Parameters | 605 | 332 | 347 | 353 |
| R (int) | 0.0206 | 0.0604 | 0.0210 | 0.0274 |
| GOF on F2 | 1.036 | 1.061 | 1.028 | 1.049 |
| Final R indices [I ≥ 2σ(I)] | R1 = 0.0625, wR2 = 0.1349 | R1 = 0.0689, wR2 = 0.1805 | R1 = 0.0688, wR2 = 0.1755 | R1 = 0.0805, wR2 = 0.2122 |
| Salts | 9 | 10 | 11 | 12 |
|---|---|---|---|---|
| Empirical formula | C60H68N2O10S2 | C58H60N2O12S2 | C56H58N4O10S2 | C54H56N2O10S4 |
| Formula weight | 1041.28 | 1041.20 | 1011.18 | 1021.25 |
| Space group | C2 | P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
| a/Å | 25.6094(14) | 9.0753(8) | 10.6098(10) | 8.9446(8) |
| b/Å | 8.1885(3) | 13.0083(9) | 12.0437(9) | 11.9700(10) |
| c/Å | 15.2041(7) | 13.1464(12) | 12.3814(8) | 12.7475(8) |
| α/° | 90.00 | 63.421(8) | 64.981(7) | 103.421(6) |
| β/° | 118.790(4) | 75.925(8) | 78.038(7) | 107.836(7) |
| γ/° | 90.00 | 72.752(7) | 66.971(8) | 94.705(7) |
| V/Å−3 | 2794.2(2) | 1314.42(19) | 1317.73(18) | 1246.36(17) |
| Z | 2 | 1 | 1 | 1 |
| Dc/g m−3 | 1.238 | 1.315 | 1.274 | 1.361 |
| μ (Mo Kα)/mm−1 | 0.155 | 0.167 | 0.163 | 0.253 |
| F(000) | 1108 | 550 | 534 | 538 |
| Reflections collected | 5178 | 7815 | 7789 | 7453 |
| Unique reflections | 4586 | 6004 | 6013 | 5697 |
| Parameters | 374 | 346 | 339 | 330 |
| R (int) | 0.0208 | 0.0167 | 0.0168 | 0.0154 |
| GOF on F2 | 1.022 | 1.038 | 1.024 | 1.035 |
| Final R indices [I ≥ 2σ(I)] | R1 = 0.0608, wR2 = 0.1504 | R1 = 0.0663, wR2 = 0.1626 | R1 = 0.0522, wR2 = 0.1228 | R1 = 0.0538, wR2 = 0.1404 |
| D–H⋯A | d(D–H) | d(H⋯A) | d(D⋯A) | <(DHA) |
|---|---|---|---|---|
| a Symmetry operations: for 2(H3O)+·(H2M)2−, i −x + 1, y + 1, −z + 1/2; ii −x + 1, y, −z + 1/2; iii −x + 3/2, −y + 1/2, −z + 1; iv −x + 1/2, y − 1/2, −z + 1/2; v x, y − 1, z; vi −x + 1, −y, −z + 1. For 1, i −x, −y + 1, −z; ii −x + 1, −y + 2, −z; iii x, y − 1, z; iv −x + 1, −y + 1, −z; v x, y + 1, z. For 2, ii −x + 1, y, −z + 1/2; iii x, −y + 1, z + 1/2; iv −x + 1, −y + 1, −z. For 3, i −x + 1, −y + 3, −z; ii −x + 1, −y + 2, −z; iii x, y − 1, z; iv x, y + 1, z. For 4, ii x + 1, y, z; iii −x, −y + 1, −z. For 5, i −x + 1, −y, −z + 1; ii −x + 1, −y, −z + 2. For 6, ii −x, −y + 2, −z + 1. For 7, ii x, y + 1, z; iii −x + 1/2, y + 1/2, −z + 1/2. For 8, ii x, y + 1, z; iii −x + 3/2, y + 1/2, −z + 2. For 9, ii x, y − 1, z; iii −x + 1/2, y − 1/2, −z + 1. For 10, iv −x + 1, −y, −z + 1. For 11, ii −x + 2, −y, −z + 2. For 12, ii −x + 1, −y + 1, −z + 1. | ||||
| 2(H3O)+·(H2M)2− | ||||
| O(1W)–H(1W1)⋯O(5)i | 0.849(9) | 2.155(11) | 2.990(3) | 168(2) |
| O(1W)–H(1W2)⋯O(6)ii | 0.851(9) | 2.286(17) | 3.034(3) | 147(2) |
| O(1W)–H(1W2)⋯O(3)iii | 0.851(9) | 2.47(2) | 3.003(3) | 121(2) |
| O(1W)–H(1W3)⋯O(3) | 0.846(9) | 1.945(10) | 2.777(3) | 167(3) |
| O(2W)–H(2W3)⋯O(6)iv | 0.867(10) | 2.101(19) | 2.879(3) | 149(3) |
| O(2W)–H(2W1)⋯O(6)v | 0.864(10) | 1.905(10) | 2.767(3) | 175(4) |
| O(2W)–H(2W2)⋯O(2)vi | 0.872(10) | 2.007(17) | 2.835(3) | 158(3) |
| O(4)–H(4O)⋯O(7)i | 0.845(10) | 1.885(11) | 2.726(3) | 173(4) |
| O(8)–H(8O)⋯O(1)vi | 0.841(10) | 1.838(16) | 2.645(3) | 161(4) |
| 1 | ||||
| O(1W)–H(2W1)⋯O(3) | 0.85(5) | 2.24(7) | 2.904(5) | 135(8) |
| O(2W)–H(1W2)⋯O(1W)i | 0.86(5) | 2.03(3) | 2.822(6) | 151(4) |
| O(2W)–H(2W2)⋯O(3W)ii | 0.86(5) | 2.66(5) | 3.072(4) | 111(4) |
| O(3W)–H(1W3)⋯O(5)ii | 0.85(5) | 1.891(11) | 2.723(3) | 165(3) |
| O(3W)–H(2W3)⋯O(7) | 0.85(5) | 1.971(15) | 2.778(3) | 160(3) |
| O(4)–HO4⋯O(2) | 0.85(5) | 1.944(19) | 2.716(3) | 151(3) |
| O(4W)–H(1W4)⋯O(2) | 0.85(5) | 2.025(15) | 2.851(3) | 164(4) |
| O(4W)–H(2W4)⋯O(7)iii | 0.85(5) | 2.15(2) | 2.862(3) | 142(3) |
| O(8)–HO8⋯O(1)iv | 0.85(5) | 2.010(15) | 2.815(2) | 159(3) |
| N(1)–H(1N1)⋯O(1) | 0.87(3) | 2.244(17) | 3.008(3) | 147(2) |
| N(1)–H(1N2)⋯O(3W)iii | 0.86(3) | 1.979(10) | 2.835(3) | 175(2) |
| N(1)–H(1N3)⋯O(4W) | 0.86(3) | 1.971(10) | 2.834(3) | 176(2) |
| N(2)–H(2N1)⋯O(6) | 0.87(3) | 1.958(9) | 2.821(3) | 176(2) |
| N(2)–H(2N2)⋯O(3)v | 0.87(3) | 2.252(12) | 3.095(3) | 163(2) |
| N(2)–H(2N3)⋯O(2W) | 0.86(3) | 1.970(11) | 2.825(3) | 170(2) |
| 2 | ||||
| O(1W)–H(1W)⋯O(1)ii | 0.85(3) | 2.12(2) | 2.904(3) | 153(3) |
| O(1W)–H(2W)⋯O(2)iii | 0.85(3) | 2.176(18) | 2.985(3) | 158(3) |
| O(4)–H(4O)⋯O(3) | 0.85(3) | 1.94(2) | 2.712(3) | 150(4) |
| N(1)–H(1N1)⋯O(3) | 0.87(3) | 1.981(10) | 2.852(3) | 178(2) |
| N(1)–H(1N2)⋯O(1W) | 0.87(3) | 1.882(11) | 2.754(3) | 175(3) |
| N(1)–H(1N3)⋯O(2)iv | 0.86(3) | 1.997(16) | 2.801(3) | 155(2) |
| 3 | ||||
| O(1W)–H(1W)⋯O(3)i | 0.85(3) | 2.121(19) | 2.874(3) | 148(3) |
| O(1W)–H(1W)⋯O(4)i | 0.85(3) | 2.45(3) | 3.071(3) | 130(3) |
| O(1W)–H(2W)⋯O(2) | 0.85(3) | 1.910(15) | 2.719(3) | 158(3) |
| O(4)–H(4O)⋯O(5)ii | 0.85(3) | 1.961(11) | 2.811(2) | 176(3) |
| O(8)–H(8O)⋯O(7) | 0.85(3) | 1.853(17) | 2.645(3) | 154(3) |
| O(9)–H(9O)⋯O(3)iii | 0.85(3) | 2.13(2) | 2.852(3) | 142(3) |
| O(10)–H(10O)⋯O(1W) | 0.85(3) | 1.942(13) | 2.789(3) | 171(4) |
| N(1)–H(1N1)⋯O(10) | 0.87(3) | 1.966(10) | 2.828(3) | 174(2) |
| N(1)–H(1N2)⋯O(1) | 0.87(3) | 1.962(9) | 2.827(2) | 173.6(19) |
| N(1)–H(1N3)⋯O(7)iv | 0.87(3) | 1.963(10) | 2.829(2) | 175(2) |
| N(2)–H(2N1)⋯O(9) | 0.87(3) | 1.947(10) | 2.807(3) | 172(2) |
| N(2)–H(2N2)⋯O(6) | 0.87(3) | 1.984(16) | 2.775(3) | 151(2) |
| N(2)–H(2N3)⋯O(1W)ii | 0.86(3) | 1.978(10) | 2.836(3) | 174(2) |
| 4 | ||||
| O(4)–H(4O)⋯O(3) | 0.85(3) | 2.00(2) | 2.776(3) | 151(3) |
| O(5)–H(5O)⋯O(6) | 0.85(3) | 1.80(2) | 2.616(4) | 161(5) |
| O(6)–H(6O)⋯O(3)ii | 0.85(3) | 1.95(3) | 2.745(4) | 155(6) |
| N(1)–H(1N1)⋯O(2) | 0.87(2) | 1.986(10) | 2.855(2) | 176(2) |
| N(1)–H(1N2)⋯O(5) | 0.87(2) | 1.873(10) | 2.744(3) | 171(2) |
| N(1)–H(1N3)⋯O(1)iii | 0.87(2) | 1.957(9) | 2.825(2) | 177(2) |
| 5 | ||||
| O(4)–H(4O)⋯O(2) | 0.85(2) | 1.88(2) | 2.683(3) | 155(5) |
| O(8)–H(8O)⋯O(7) | 0.85(2) | 1.951(16) | 2.783(4) | 164(4) |
| O(9)–H(9O)⋯O(2) | 0.85(2) | 2.112(16) | 2.922(3) | 160(3) |
| N(1)–H(1N1)⋯O(3) | 0.86(3) | 1.908(14) | 2.745(3) | 162(3) |
| N(1)–H(1N2)⋯O(9) | 0.86(3) | 2.142(16) | 2.957(3) | 157(3) |
| N(1)–H(1N3)⋯O(1)i | 0.86(3) | 1.920(11) | 2.775(3) | 170(2) |
| N(2)–H(2N1)⋯O(5) | 0.86(3) | 1.961(10) | 2.821(3) | 175(3) |
| N(2)–H(2N2)⋯O(6)ii | 0.86(3) | 1.937(11) | 2.787(3) | 168(2) |
| N(2)–H(2N3)⋯O(9)i | 0.86(3) | 2.139(12) | 2.980(3) | 165(2) |
| 6 | ||||
| O(4)–H(4O)⋯O(2) | 0.85(3) | 2.00(3) | 2.777(4) | 150(5) |
| O(5)–H(5O)⋯O(1W) | 0.82 | 1.92 | 2.734(11) | 174.0 |
| N(1)–H(1N1)⋯O(1)ii | 0.86(3) | 1.960(10) | 2.828(3) | 178(2) |
| N(1)–H(1N2)⋯O(3) | 0.86(3) | 1.940(10) | 2.801(3) | 175(2) |
| N(1)–H(1N3)⋯O(5) | 0.86(3) | 1.929(14) | 2.776(4) | 167(3) |
| 7 | ||||
| O(4)–H(4O)⋯O(3) | 0.85(4) | 1.93(2) | 2.708(4) | 152(4) |
| O(5)–H(5O)⋯O(1)ii | 0.85(4) | 1.85(2) | 2.674(5) | 163(6) |
| N(1)–H(1N1)⋯O(3) | 0.86(4) | 1.929(12) | 2.769(4) | 164(2) |
| N(1)–H(1N2)⋯O(5) | 0.86(4) | 1.933(13) | 2.782(4) | 168(3) |
| N(1)–H(1N3)⋯O(2)iii | 0.86(4) | 1.981(11) | 2.829(4) | 167(2) |
| 8 | ||||
| O(4)–H(4O)⋯O(3) | 0.85(3) | 1.89(3) | 2.702(8) | 160(8) |
| O(5)–H(5O)⋯O(2)ii | 0.82 | 1.94 | 2.714(9) | 156.4 |
| N(1)–H(1N1)⋯O(3) | 0.85(2) | 1.99(2) | 2.782(8) | 153(4) |
| N(1)–H(1N2)⋯O(5) | 0.85(2) | 1.955(17) | 2.793(7) | 169(5) |
| N(1)–H(1N3)⋯O(1)iii | 0.86(2) | 1.99(2) | 2.813(7) | 161(5) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| 9 | ||||
| O(4)–H(4O)⋯O(3) | 0.85(3) | 1.96(3) | 2.736(8) | 151(6) |
| O(5)–H(5O)⋯O(2)ii | 0.85(3) | 1.98(6) | 2.683(9) | 139(8) |
| N(1)–H(1N1)⋯O(3) | 0.86(3) | 1.999(19) | 2.804(8) | 155(3) |
| N(1)–H(1N2)⋯O(5) | 0.86(3) | 1.912(13) | 2.769(6) | 175(4) |
| N(1)–H(1N3)⋯O(1)iii | 0.86(3) | 2.004(19) | 2.824(7) | 158(4) |
| 10 | ||||
| O(4)–H(4O)⋯O(2) | 0.85(3) | 1.84(2) | 2.649(3) | 158(5) |
| N(1)–H(1N1)⋯O(5) | 0.87(3) | 2.019(13) | 2.862(3) | 163(2) |
| N(1)–H(1N2)⋯O(2) | 0.87(3) | 2.127(10) | 2.980(3) | 171(2) |
| N(1)–H(1N2)⋯O(1) | 0.87(3) | 2.57(2) | 3.146(3) | 125(2) |
| N(1)–H(1N3)⋯O(1)iv | 0.87(3) | 1.894(11) | 2.751(3) | 169(2) |
| 11 | ||||
| O(4)–H(4O)⋯O(2) | 0.85(2) | 1.854(16) | 2.671(2) | 160(3) |
| N(1)–H(1N1)⋯O(3) | 0.87(2) | 1.893(9) | 2.766(2) | 175.5(18) |
| N(1)–H(1N2)⋯O(2)ii | 0.86(2) | 2.020(9) | 2.879(2) | 175.5(16) |
| N(1)–H(1N3)⋯O(5) | 0.87(2) | 1.910(11) | 2.753(2) | 163.0(17) |
| 12 | ||||
| O(4)–H(4O)⋯O(1) | 0.85(3) | 1.853(18) | 2.654(3) | 156(4) |
| N(1)–H(1N1)⋯O(5) | 0.86(3) | 1.836(10) | 2.695(3) | 173(2) |
| N(1)–H(1N2)⋯O(1) | 0.86(3) | 2.097(11) | 2.923(3) | 161.4(19) |
| N(1)–H(1N3)⋯O(2)ii | 0.86(3) | 1.997(13) | 2.825(3) | 162(2) |
Results and discussion
Experimental strategy
To modulate the supramolecular patterns and luminescent properties of target molecules, three strategies are concerned in our present experiment. First, the identical C3v symmetry of –SO3− and –NH3+ groups could lead to the formation of high symmetrical supramolecular patterns despite of the different pendant groups.7c Therefore, how to break the regular hydrogen bonds between –SO3− and –NH3+ groups to achieve the aim of modulating supramolecular architectures will be an interesting subject. Therefore, hydroxyl groups are introduced to the ortho-position of –SO3− groups. The two hydroxyl groups act as both donor and acceptor to involve in the formation of hydrogen bonding interactions, thus reducing the C3v symmetry of –SO3− and further modulating the supramolecular network of target molecules. Second, from the viewpoint of synthesis, solvent molecules with different volume, carbochain length, and diverse hydrogen bonding donors and acceptors would be good candidates owing to their different abilities for building hydrogen bonds. Subsequently, nine kinds of solvent solutions, water, methanol, ethanol, n-propanol, n-butanol, n-pentanol, 1,4-dioxane, dimethylformamide and dimethyl sulfoxide, were selected as reaction medium to study their effects on the supramolecular patterns and luminescent properties of organic salts comprising H4M and TPMA. As anticipated, different volume, carbochain length, and types of donors and acceptors of solvents lead to the formation of diverse supramolecular patterns. Third, assemble process also has large effect on the structures and properties of salts.11 As a result, general and grinding methods are employed to investigate such effect. Herein, the assemble process influence on the structure is, in fact, the effects of the solvent molecules. That is, small H2O and MeOH solvent molecules form different structures in 1 and 2, 3 and 4 under general and grinding method. Large solvent molecules, such as ethanol, n-propanol, n-butanol, n-pentanol, 1,4-dioxane, dimethylformamide and dimethyl sulfoxide, usually present the same structures (6–12). The results indicate that the grinding method tends to form structures with little or no H2O molecules. Interestingly, after being exposed to the air for several hours, a new salt 5 could be obtained from the transformation of salt 4. Meanwhile, the trans conformation of H2M2− dianions in 4 also transferred into cis conformation in 5.Crystal structure of 2(H3O)+·(H2M)2−
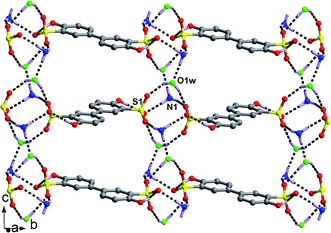
Recrystallization of H4M in aqueous solution leads to the formation of 2(H3O)+·(H2M)2− (Fig. S1†). The components connect with each other through O–H⋯O hydrogen bonding interactions (Table 2), thus generating a 3-D supramolecular network (Fig. 1c) which can be understood as the following manners. For the organic parts, two H2M2− dianions join together through hydrogen bonding interactions between sulfonate O1 and hydroxyl O8 to form dimer unit containing R22(22) ring (Fig. 1a). Adjacent units interlink with each other through hydrogen bonding interactions between sulfonate O7 and hydroxyl O4, giving rise to layer motif in the bc plane which contain large R66(46) ring (Fig. 1a). Interestingly, the dimers in one such layer insert into the large 46-membered rings of another identical layer, forming 2-fold interpenetrating network as shown in Fig. 1b. The distance between two nearest phenyl rings in the interpenetrating network is 5.049(1) Å. For the inorganic part, H3O1w+ cations connect adjacent –S1O3 and –S2O3 groups at the points of O3, O5 and O6 to form chain structure along b-axis, which are further joined by the H3O2w+ cations at the point of O2, O6 and O6i to generate tape structure (Fig. 1d). Adjacent tapes connect with each other through the hydrogen bonds between O3 and H3O1w+ cations, also generating layer motif (Fig. 1d). Combination of organic and inorganic layers leads to the formation of 3-D supramolecular network (Fig. 1c). It should be noted that the involving of hydroxyl groups in the hydrogen bonding interactions effectively reduces the C3v symmetry of –SO3−, so that the sulfonate groups could not form regular patterns with H3O+ cations.Crystal structures of salts 1–12
Single-crystal X-ray analyses indicate that crystal structures of salts 1–12 involve different components. As illustrated in Fig. S1,† all the crystal structures of salt 1–12 are containing 2(HTPMA)+·(H2M)2− pairs and different solvent molecules, which interact with each other by N–H⋯O or O–H⋯O hydrogen bonding interactions. For salt 10, additional C–H⋯O hydrogen bond (C12–H12A⋯O6, 2.743(2) Å) between one of the DO molecules and the phenyl ring of HTPMA+ cation is also observed. The two –SO3 groups of H2M2− dianions in these salts adopt trans conformation except for salt 5 (Scheme 1). The different volume and ability of forming hydrogen bonding interactions of series of solvent molecules induces multiple supramolecular patterns formed by –SO3 and –NH3 groups, which then lead to diverse supramolecular architectures in these eleven salts.In salt 1, the four different H2O molecules play different role during the assemble process. For O1w and O2w, two of them connect two –N2H3 groups and two sulfonate O3 atoms through hydrogen bonding interactions, forming finite [(SO3)2(NH3)2(H2O)4] cluster with R68(16) ring (Fig. 2). Meanwhile, a pair of O3w, O4w, –N1H3, –S1O3 and –S2O3 groups interact with each other through hydrogen bonding interactions to form finite [(SO3)4(NH3)2(H2O)4] cluster with R33(8), R34(8) and R44(12) rings (Fig. 2). Then, the two types of clusters interact with each other to generate a 1-D tape involving R68(16) rings (Scheme 2). Moreover, the biphenyl rings of the H2M2− dianions connect adjacent tapes, thus leading to the formation of (4,4) layer structure with the [(SO3)4(NH3)2(H2O)4] clusters being considered as four-connected nodes (Fig. 2).
 | ||
| Scheme 2 Diverse supramolecular patterns of the –SO3 and –NH3 groups tunned by solvent molecules in salts 1–12. | ||
Compared with salt 1, salt 2 is crystallized from the reaction of H4M and TPMA by grinding method and its unit cell consists of only two water molecules besides two HTPMA+ cations and one H2M2− dianion (Fig. S1†). Two symmetry-related –SO3 groups and –NH3 groups interact with each other through the N–H⋯O hydrogen bonding interactions to form finite classic [(SO3)2(NH3)2] motifs, which are further joined by a pair of O1w molecules to produce chains along c-axis (Fig. 3). Then, the biphenyl rings of the H2M2− dianions extend the chains into (4,4) layer structure when the classic [(SO3)2(NH3)2] motifs are considered as four-connected nodes (Fig. 3).
Evaporation of H4M and TPMA in methanol solution leads to the formation of salt 3, which contains two kinds of solvent molecules, i.e. two MeOH molecules and one water molecule, in its crystal structure (Fig. S1†). As illustrated in Scheme 2, two O1w, four –SO3, four –NH3 and four MeOH molecules interact with each other through the N–H⋯O and O–H⋯O hydrogen bonding interactions to form finite [(SO3)4(NH3)4(MeOH)4(H2O)2] cluster, which incorporates hydrogen bonding rings with graph set R34(8), R44(10), R44(12) and R55(14). Different from salt 1, the lack of water molecules and poor hydrogen bonding modes of MeOH prevent the further extension of the clusters. Hence, only double chains along b-axis are formed by the interconnection of biphenyl rings of the H2M2− dianions (Fig. 4).
Salt 4 is obtained from the reaction of H4M and TPMA by grinding method. After recrystallization from MeOH solution, only four MeOH molecules exist in the crystal structure (Fig. S1†). As shown in Fig. 5, adjacent classic [(SO3)2(NH3)2] motifs are bridged by two pair of different MeOH molecules (O5 and O6) to generate 1-D tapes along a-axis. Such interconnection gives rise to the formation of macrocycle with graph set R88(20) (Fig. 5). As the case in salt 2, the biphenyl rings of the H2M2− dianions extend the tapes into (4,4) layer structure with the classic [(SO3)2(NH3)2] motifs being considered as four-connected nodes (Fig. 5).
An interesting feature for salt 4 is that it can transform into a new salt 5 after being exposed to the air for ca. six hours. As shown in Fig. S1,† its crystal structure only contains one MeOH molecule. It should be noted that the two –SO3 groups in salt 5 adopt cis conformation (Scheme 1) and interact with HTPMA+ cations through hydrogen-bonding interactions, thus forming 1-D snake chain along the c-axis (Fig. 6). MeOH molecules are then encapsulated in the reversed grooves (Fig. 6).
Salt 6 exhibits the same space group and the similar structure motifs with salt 4 although they are obtained from different solvents. As shown in Fig. 7, the 1-D tapes along a-axis in salt 6 are bridged by one pair of water molecules and one pair of EtOH molecules instead of two pair of different MeOH molecules in salt 4. Owing to the similar tape structure, salt 6 also exhibits (4,4) layer structure with the classic [(SO3)2(NH3)2] motifs being considered as four-connected nodes and the biphenyl rings of the H2M2− dianions being considered as linkers (Fig. 7).
As the case in salts 4 and 5, salts 7–9 also present similar structure motifs despite they crystallize from different n-propanol, n-butanol and n-pentanol solution. As shown in Scheme 2, the –SO3 groups and –NH3 groups in the three salts interact with each other through the N–H⋯O hydrogen bonding interactions between two oxygen and two hydrogen atoms to generate zig-zag chain along the b-axis. The left oxygen and hydrogen atoms form N–H⋯O and O–H⋯O hydrogen bonding interactions with hydroxyl groups of n-PrOH, n-BuOH and n-PeOH molecules, thus giving rise to the formation of 1-D tapes along the same axis which incorporate continuous R55(14) rings. The 1-D tapes are further extended into (6,3) layer structure by the biphenyl rings of the H2M2− dianions with the single [(SO3)(NH3)] motif being viewed as three-connected nodes (Fig. 8). It is interesting to note that these (6,3) layers contain hexagonal windows with the diagonal distances (two opposite S atoms) in the three salts are 12.2 × 12.2 × 16.5 Å3 (7), 12.2 × 12.2 × 16.7 Å3 (8) and 12.3 × 12.3 × 16.9 Å3 (9), respectively. Such windows are too large so that they can accommodate a pair of n-PrOH, n-BuOH and n-PeOH molecules (Fig. 8).
Two symmetry-related –SO3 groups and two symmetry-related –NH3 groups in salt 10 interact with each other through the N–H⋯O hydrogen bonding interactions to form finite [(SO3)2(NH3)2] motif as shown in Scheme 2. Different from the [(SO3)2(NH3)2] motif in salts 4 and 6, the present one contains a small hydrogen bonding ring described by the graph set R24(8). Then, one of the two DO molecules acts as bridge to connect adjacent [(SO3)2(NH3)2] motifs at the points of H1N1 with the two acceptor oxygen atoms, thus generating chain structure along the a-axis (Scheme 2). Moreover, the biphenyl rings of the H2M2− dianions extend the chains into (4,4) layer structure when the [(SO3)2(NH3)2] motifs are considered as four-connected nodes (Fig. 9).
Salt 11 and 12 are obtained from different DMF and DMSO solvents, however, they show the similar structure motifs owing to single acceptor oxygen atom they have. As shown in Scheme 2, two symmetry-related –SO3 groups and two symmetry-related –NH3 groups interact with each other through the N–H⋯O hydrogen bonding interactions to form finite classic [(SO3)2(NH3)2] motif with R44(12) ring. The left hydrogen atoms of –NH3 groups interact with the oxygen atoms of DMF and DMSO molecules by the N–H⋯O hydrogen bonding interactions, generating [(SO3)2(NH3)2(solvent)2] (solvent = DMF and DMSO) clusters. In contrast to the double acceptor in DO molecule, the single acceptor can only afford the formation of chain structure which forms by the interlinking of biphenyl rings of the H2M2− dianions and [(SO3)2(NH3)2] clusters (Fig. 10).
Diverse ⋯(–SO3)⋯(–NH3)⋯(solvent)⋯ patterns induced by different solvents
Both –SO3 and –NH3 groups have three active sites to form hydrogen bonding interactions, which then generate multiple hydrogen bonding patterns according to the different chemical environments. For clarity, the hydrogen bonding modes of the –SO3 and –NH3 groups are denoted as AnAnAn (A = O or H, n = 0 and 1).8 As shown in Scheme 2, diverse ⋯(–SO3)⋯(–NH3)⋯(solvent)⋯ patterns could be obtained due to the different character of the nine solvent molecules. Indeed, these solvent molecules could be generally divided into two series according to their roles in forming hydrogen patterns: (I) acting as donor and acceptor, such as H2O, MeOH, EtOH, n-PrOH, n-BuOH, and n-PeOH; (II) acting as acceptor, such as DO, DMF, and DMSO.For the first series, five kinds of patterns are observed owing to the different volume and carbochain length of solvents. In salt 1, four –SO3 groups, four –NH3 groups and eight water molecules connect with each other to generate [(SO3)4(NH3)4(H2O)8] cluster, in which the –SO3 and –NH3 groups present A1A1A2 and A1A1A1 hydrogen bonding modes. Then, the terminal O1w and O2w molecules extend adjacent clusters into chair motifs. In contrast, although the –SO3 and –NH3 groups in salt 3 present the same A1A1A2 and A1A1A1 hydrogen bonding modes, only discrete [(SO3)4(NH3)4(MeOH)4(H2O)2] cluster is formed. This is mainly ascribed to the lack of water molecules and poor hydrogen bonding modes of MeOH which prevent the further extension of the clusters. Two –SO3 and two –NH3 groups in salt 2 exhibit A1A1A0 hydrogen bonding mode and interact with each other through hydrogen bonding interactions to generate classic [2 + 2] rings described by the graph set R44(12), which are then connected by a pair of water molecules at the points of left oxygen and hydrogen atoms to generate chain structure along the c-axis. Totally, the –SO3 and –NH3 groups in this salt exhibit the same A1A1A1 hydrogen bonding mode in consideration of the participation of solvent molecules, however, the different amount and diverse hydrogen bonding modes of water molecules in salts 1 and 2 result in obvious distinction. Salts 4 and 6, isolated from different methanol and ethanol solution, exhibit the similar 1-D tape, in which the –SO3 and –NH3 groups have the same A1A1A1 hydrogen bonding modes as those in salt 2. Similarly, two –SO3 and two –NH3 groups exhibit A1A1A0 hydrogen bonding mode and interact with each other through hydrogen bonding interactions to generate classic [2 + 2] rings described by the graph set R44(12). Instead of the water molecules in salt 2, two MeOH molecules in salt 4, one H2O and one EtOH molecule in 5 act as bridge to join adjacent [2 + 2] rings, thus forming the 1-D tape along the a-axis. As result of the longer bridge in these two salts, macrocycle R88(20) rings take the place of R46(12) ring in salt 2. As the case in salts 4 and 6, two –SO3 groups and two –NH3 groups in salt 5 present the same A1A1A0 hydrogen bonding modes and connect with each other to generate classic [2 + 2] ring. Then, single MeOH molecule instead of four solvent molecules in salts 4 and 6 extend adjacent rings into chain structure. Different from the discrete [(SO3)2(NH3)2] in the above five salts, the –SO3 and –NH3 groups in salts 7–9 connect with each other in the same A1A1A0 hydrogen bonding mode to give rise to zig-zag chains. The solvent molecules (n-PrOH, n-BuOH and n-PeOH) act as both donor and acceptor and interact with the left oxygen and hydrogen atoms to form 1-D tapes which incorporate continuous R55(14) rings. Therefore, the total hydrogen bonding modes of –SO3 and –NH3 groups is A1A1A1 as observed in salts 1, 4 and 6.
For the second series, two kinds of patterns are observed owing to the different amount of acceptor oxygen atoms in the three solvents. In salt 10, two –SO3 groups and two –NH3 groups interact with each other in the same A2A1A0 hydrogen bonding modes to form different [(SO3)2(NH3)2] motif from the classic one. This new motif contains a small hydrogen bonding ring described by the graph set R24(8). The DO molecule has two accepter oxygen atoms in the para position, which makes the DO molecule a potential bridge. Therefore, the two oxygen atoms form hydrogen bonding interactions with the left hydrogen atoms of –NH3 groups, extending adjacent [(SO3)2(NH3)2] motifs into chain structure along the a-axis. Such connection gives rise to the different A2A1A1 hydrogen bonding mode for the –NH3 group. By contrast, the –SO3 and –NH3 groups in salts 11 and 12 exhibit A1A1A0 hydrogen bonding modes and connect with each other in pairs to form the classic [(SO3)2(NH3)2] motif that incorporates R44(12) ring. It is noteworthy that the [(SO3)2(NH3)2] motif in salts 11 and 12 can not be extended to chain structure by solvent molecules because there is only one acceptor oxygen atom in DMF and DMSO molecules. Instead, a pair of these two kinds of molecules attach to the [(SO3)2(NH3)2] motif through hydrogen bonding interactions at the points of left hydrogen atoms, thus forming [(SO3)2(NH3)2(solvent)2] (solvent = DMF and DMSO) cluster with the –NH3 group exhibiting A1A1A1 mode. It can be concluded that solvent molecules have important effect on the supramolecular patterns formed by –SO3 and –NH3 groups, which then certainly result in diverse packing diagram.
Packing diagram tuned by diverse solvent molecules
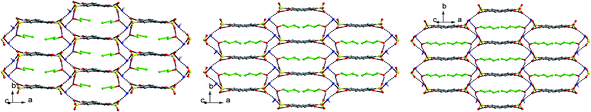
From the viewpoint of crystal engineering, solvent molecules with different spatial structure, carbochain length, number of donor and acceptor play important roles during the self-assemble process of supramolecules and will obviously influence the packing diagram of the obtained salts. In this article, nine kinds of solvents are employed to investigate their effect on the supramolecular structures. Interestingly, structural analyses indicate that five types of packing diagrams are formed with the above solvents (Scheme 3, Fig. S2†), which are in accordance with the evolution of ⋯(–SO3)⋯(–NH3)⋯(solvent)⋯ patterns.In salts 1 and 3, pairs of HTPMA+ cations arrange in tail-to-tail mode (Scheme 3, Fig. S2†) to form column motifs along the b-axis which extend the layers of H2M2− dianions into pillared layered supramolecular network (Type I). The different H2O and MeOH molecules fill in the channels formed by adjacent column pillars. On the contrary, pairs of HTPMA+ cations in salt 2 arrange in head-to-head mode (Scheme 3) and form layer structures together with pairs of H2M2− dianions through N–H⋯O and O–H⋯O hydrogen bonding interactions. It is interesting to note that the template effect of H2O molecules results in the formation of the regular channels along the c-axis. Therefore, the O1w molecules are encapsulated in these channels while the disordered O2w molecules lie between biphenyl rings of the H2M2− dianions. Packing of adjacent layers leads to the formation of Type II structure for salt 2. In salts 4 and 6, the HTPMA+ cations and H2M2− dianions are all alternately arranged along c-axis, thus forming a column motif. Such arrangement affords the formation of continuous side-to-plane and plane-to-plane π⋯π interactions between HTPMA+ cations and H2M2− dianions in these two salts (Fig. S3†). The centroid-to-centroid distances are 3.968(2) (plane-to-plane) and 3.773(3) Å (side-to-plane) in salt 4 and 3.885(9) (plane-to-plane) and 3.795(1) Å (side-to-plane) in salt 6. Then, the packing of adjacent columns leads to interlayer space in the two salts which filled by the MeOH molecules in salt 4, and EtOH, H2O molecules in salt 6. Owing to the similar sizes of the space induced by the different solvents, the two salts present the similar packing diagram, Type III. The HTPMA+ cations in salt 5 arrange along the a-axis in tail-to-tail mode (Scheme 3) to form column motifs which are then sandwiched between biphenyl rings instead of the –SO3 groups, generating layer structure. The packing of adjacent layers affords the formation of Type IVA packing diagram. Salts 7–9 present the same packing diagram (Type V), in which pairs of head-to-head HTPMA+ cations (Scheme 3, Fig. S2†) are sandwiched between the –SO3 groups through hydrogen bonding interactions, generating (6,3) layer structure as illustrated in Fig. 8. Then, arrangement of adjacent (6,3) layers gives rise to a graphite-like structure. Interestingly, the n-PrOH, n-BuOH and n-PeOH molecules locate in the lamella through hydrogen bonding interactions with –NH3 groups rather than fill in the interlayer space. In comparison with the head-to-head mode of HTPMA+ cations in salts 7–9, the HTPMA+ cations in salts 10–12 arrange along the a-axis in tail-to-tail mode (Scheme 3, Fig. S2†) to form similar packing diagram with that of salt 5. It should be noted that side-to-plane π⋯π interactions between HTPMA+ cations and H2M2− dianions in salts 10 and 12 are observed with the centroid-to-centroid distances being 3.907(4) and 3.993(3) Å (Fig. S4†). Subsequently, the packing of adjacent layers affords the formation of Type IVB packing diagram in salts 10 and 12 and Type IVC packing diagram in salt 11. For salts 10 and 12, the DO and DMSO molecules fill in the narrow interlayer space. However, the DMF molecules in salt 11 locate in the lamella through hydrogen bonding interactions with –NH3 groups. In a word, tuning the packing diagram of the salts through the alternation of solvent molecules is successfully achieved in the present eleven salts.
IR spectroscopy
The stretching bands around 3452–3276 cm−1 in the IR spectra of salts 1–12, as well as the broad and strong stretching bands appearing in the range of 3091–2848 cm−1, can be attributed to the existence of the hydroxyl groups, primary ammonium cations, water molecules, as well as the formation of extensive hydrogen bonding interactions. The bands appearing in the range of 1614–1446 cm−1 and around 1380 cm−1 in salts 1–12 are ascribed to the skeletal vibration of aromatic rings and νC–O(phenol) stretching vibrations, respectively. Meanwhile, the peak at 1662 cm−1 in salt 11 can be attributed to the νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration of the DMF molecule. The IR spectra of these crystals clearly exhibit the characteristic vibrations of νas(SO3−) which locate at the range of 1257–1153 cm−1, whereas the νs(SO3−) absorptions are at the range of 1095–1014 cm−1.8,12
O stretching vibration of the DMF molecule. The IR spectra of these crystals clearly exhibit the characteristic vibrations of νas(SO3−) which locate at the range of 1257–1153 cm−1, whereas the νs(SO3−) absorptions are at the range of 1095–1014 cm−1.8,12
Luminescent property
The luminescent properties of 2(H3O)+·(H2M)2−, salts 1–12 and the reactants in the solid state at room temperature were investigated. As shown in Fig. 11, upon excitation at 310 nm, 2(H3O)+·(H2M)2− exhibits the emission maximum at 374 nm while TPMA molecule exhibits weak emission maximum at 423 nm (λex = 345 nm) (Fig. S7†). All the emissions of the complexes could be attributed to the π–π* transitions.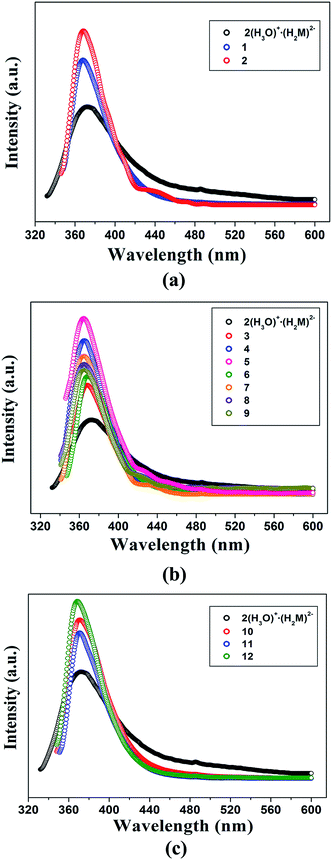 | ||
| Fig. 11 Emission spectra of 2(H3O)+·(H2M)2−, 1, 2 (a), 3–9 (b) and 10–12 (c) in the solid-state at room temperature. | ||
In view of the nature of these nine solvent molecules, the luminescent properties of salts 1–12 will be discussed in three groups: H2O, five aliphatic alcohols and three hydrogen bonding acceptor molecules (DO, DMF, and DMSO). In each groups, the properties of the solvent molecules play crucial roles on the emission intensity. As shown in Fig. 11a, upon similar excitations, the emission spectra of salts 1 and 2 in the first group exhibit similar shape and maximum at 368 (λex = 328 nm) and 369 (λex = 326 nm). Their emission intensity shows regular increase from salt 1 to salt 2 with the decrease of water molecules. That is, more water molecules tend to form more hydrogen bonds, which then increase the loss of energy.8b For 2(H3O)+·(H2M)2−, owing to the existence of the strong acidic H protons, the luminescent emission intensity is relatively lower than that of salts 1 and 2.12e In the second group, the emission maximum for salts 3–9 appears at 368 (λex = 333 nm), 365 (λex = 321 nm), 365 nm (λex = 326 nm), 369 (λex = 327 nm), 365 (λex = 321 nm), 365 (λex = 321 nm) and 365 nm (λex = 321 nm) upon similar excitations. With the increase of the carbochain, the thermal vibrations of solvent carbochains gradually intensify from MeOH in 4 to n-PeOH in 9. Therefore, partial excited electrons are quenched through the non-radioactive transition, which then leads to the decline of emission intensities.8b Subsequently, the emission intensity is gradually decreased with the increasing of the carbochain. In this sense, the emission intensity follows the order 4 > 7 > 8 > 9 (Fig. 11b). Salt 5 exhibits stronger emission intensity than that of salt 4, which follows the order in salts 1 and 2. An exception in this series is the weaker emission of salts 3 and 6, which can be attributed to water molecules involved in the two salts. Fig. 11c presents the emission spectra of the third group, in which salts 10–12 exhibit similar shape and maximum at 371 (λex = 328 nm), 371 (λex = 331 nm) and 370 nm (λex = 328 nm), respectively. In comparison with the lower intensity for salt 12, in salts 10 and 12, the side-to-plane π⋯π interactions among adjacent HTPMA+ cations and H2M2− dianions increase the mobility of the electrons, which can enhance the emission intensity.14 Especially, salt 12 exhibits the strongest emission in the three salts due to large charge density of S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group. Owing to the different structures induced by different noncovalent interactions in these three salts, their luminescent properties exhibit an inverse order to the rule that high polar solvents usually cause red-shift and decreasing of emission intensity.7e From the above discussions, solvent molecules can modulate the luminescent property of supramolecular salts except for their architectures.
O group. Owing to the different structures induced by different noncovalent interactions in these three salts, their luminescent properties exhibit an inverse order to the rule that high polar solvents usually cause red-shift and decreasing of emission intensity.7e From the above discussions, solvent molecules can modulate the luminescent property of supramolecular salts except for their architectures.
Conclusions
In summary, twelve salts assembled from 4,4′-dihydroxybiphenyl-3,3′-disulfonic acid and triphenylmethylamine in series of solvents have been synthesized and characterized. Solvent molecules with different volume and ability for building hydrogen bonds result in the formation of diverse intriguing discrete [2 + 2] motif, [4 + 4 + 6] cluster, and 1-D ⋯(–SO3)⋯(–NH3)⋯(solvent)⋯ chain and tapes, which then lead to five types of packing diagrams. Under different assemble process, small solvent molecules tend to form different structures (1 and 2, 3 and 4) while large molecules usually present the same structures (6–12). Interestingly, salts 7–9 containing longer carbochain length exhibit the same (6,3) layers with the hexagonal windows. Salt 4 can transform into a new salt 5 after being exposed to the air for several hours. The eleven salts exhibit different emission intensity with the maximum varied from 365 to 371 nm as the change of solvent molecules. The present study provides a typical instance where different assemble process can influence the structures and properties of supramolecules with preselected solvent molecules.Acknowledgements
This work is financial supported by the National Natural Science Foundation of China (51302067), Specialized Research Fund for the Doctoral Program of Higher Education of China (20132301120002), Key Project of Natural Science Foundation of Heilongjiang Province (no. ZD200903), Key Project of Education bureau of Heilongjiang Province (no. 12511z023, no. 2011CJHB006), and the Innovation team of Education bureau of Heilongjiang Province (no. 2010td03). We thank the University of Heilongjiang (Hdtd2010-04, QL201206) for supporting this study.Notes and references
-
(a) D. Yan, A. Delori, G. O. Lloyd, T. Friščić, G. M. Day, W. Jones, J. Lu, M. Wei, D. G. Evans and X. Duan, Angew. Chem., Int. Ed., 2011, 50, 12483 CrossRef CAS PubMed
; (b) T. Friščić, Chem. Soc. Rev., 2012, 41, 3493 RSC
.
-
(a) N. Schultheiss and A. Newman, Cryst. Growth Des., 2009, 9, 2950 CrossRef CAS PubMed
; (b) L. Fábían, N. Hamill, K. S. Eccles, H. A. Moynihan, A. R. Maguire, L. McCausland and S. E. Lawrence, Cryst. Growth Des., 2011, 11, 3522 CrossRef
; (c) J. I. Arenas-García, D. Herrera-Ruiz, K. Mondragón-Vásquez, H. Morales-Rojas and H. Höpfl, Cryst. Growth Des., 2012, 12, 811 CrossRef
.
-
(a) A. M. Pivovar, K. T. Holman and M. D. Ward, Chem. Mater., 2001, 13, 3018 CrossRef CAS
; (b) K. T. Holman, A. M. Pivovar and M. D. Ward, Science, 2001, 294, 1907 CrossRef CAS PubMed
; (c) K. T. Holman, A. M. Pivovar, J. A. Swift and M. D. Ward, Acc. Chem. Res., 2001, 34, 107 CrossRef CAS PubMed
; (d) R. Custelcean and M. D. Ward, Angew. Chem., Int. Ed., 2002, 41, 1724 CrossRef CAS
; (e) N. J. Burke, A. D. Burrows, M. F. Mahon and J. E. Warren, CrystEngComm, 2006, 8, 931 RSC
.
-
(a) P. C. Leverd, P. Berthault, M. Lance and M. Nierlich, Eur. J. Inorg. Chem., 2000, 133 CrossRef CAS
; (b) F. Perret, V. Bonnard, O. Danylyuk, K. Suwinska and A. W. Coleman, New J. Chem., 2006, 30, 987 RSC
; (c) X. Su, D.-S. Guo and Y. Liu, CrystEngComm, 2010, 12, 947 RSC
.
- A. M. Nowak, T. Kurc, J. Janczak and V. Videnova-Adrabinska, CrystEngComm, 2014, 16, 591 RSC
.
- V. Videnova-Adrabinska, Coord. Chem. Rev., 2007, 251, 1987 CrossRef CAS PubMed
.
-
(a) Y. Mizobe, M. Miyata, I. Hisaki, Y. Hasegawa and N. Tohnai, Org. Lett., 2006, 8, 4295 CrossRef CAS PubMed
; (b) Y. Mizobe, H. Ito, I. Hisaki, M. Miyata, Y. Hasegawa and N. Tohnai, Chem. Commun., 2006, 2126 RSC
; (c) N. Tohnai, Y. Mizobe, M. Doi, S. Sukata, T. Hinoue, T. Yuge, I. Hisaki, Y. Matsukawa and M. Miyata, Angew. Chem., Int. Ed., 2007, 46, 2220 CrossRef CAS PubMed
; (d) Y. Mizobe, T. Hinoue, A. Yamamoto, I. Hisaki, M. Miyata, Y. Hasegawa and N. Tohnai, Chem.–Eur. J., 2009, 15, 8175 CrossRef CAS PubMed
; (e) T. Hinoue, M. Miyata, I. Hisaki and N. Tohnai, Angew. Chem., Int. Ed., 2012, 51, 155 CrossRef CAS PubMed
.
-
(a) Z.-P. Deng, L.-H. Huo, H. Zhao and S. Gao, Cryst. Growth Des., 2012, 12, 3342 CrossRef CAS
; (b) Y.-N. Li, L.-H. Huo, Z.-P. Deng, X. Zou, Z.-B. Zhu, H. Zhao and S. Gao, Cryst. Growth Des., 2014, 14, 2381 CrossRef CAS
.
-
(a) D. S. Reddy, S. Duncan and G. K. H. Shimizu, Angew. Chem., Int. Ed., 2003, 42, 1360 CrossRef CAS PubMed
; (b) V. Chandrasekhar and P. Singh, Cryst. Growth Des., 2010, 10, 3077 CrossRef CAS
.
-
(a) H. S. Joshi, R. Jamshidi and Y. Tor, Angew. Chem., Int. Ed., 1999, 38, 2722 CrossRef CAS
; (b) Y. Imai, K. Murata, K. Kawaguchi, T. Sato, R. Kuroda and Y. Matsubara, Org. Lett., 2007, 9, 3457 CrossRef CAS PubMed
; (c) A. Patra and T. P. Radhakrishnan, Chem.–Eur. J., 2009, 15, 2792 CrossRef CAS PubMed
; (d) S. P. Anthony, S. Varughese and S. M. Draper, Chem. Commun., 2009, 7500 RSC
; (e) C.-P. Li and M. Du, Chem. Commun., 2011, 47, 5958 RSC
.
-
(a) Z.-P. Deng, S. Gao and L.-H. Huo, Appl. Organomet. Chem., 2007, 21, 978 CrossRef CAS
; (b) Z.-P. Deng, S. Gao, L.-H. Huo and H. Zhao, Acta Crystallogr., 2006, E62, m3230 Search PubMed
; (c) M.-Y. Shao, P. Huo, Y.-G. Sun, X.-Y. Li, Q.-Y. Zhu and J. Dai, CrystEngComm, 2013, 15, 1086 RSC
.
-
(a) Z.-P. Deng, M.-S. Li, Z.-B. Zhu, L.-H. Huo, H. Zhao and S. Gao, Organometallics, 2011, 30, 1961 CrossRef CAS
; (b) Z.-P. Deng, L.-H. Huo, M.-S. Li, L.-W. Zhang, Z.-B. Zhu, H. Zhao and S. Gao, Cryst. Growth Des., 2011, 11, 3090 CrossRef CAS
; (c) X.-Q. Fang, Z.-P. Deng, L.-H. Huo, W. Wan, Z.-B. Zhu, H. Zhao and S. Gao, Inorg. Chem., 2011, 50, 12562 CrossRef CAS PubMed
; (d) W. Wan, Z.-B. Zhu, L.-H. Huo, Z.-P. Deng, H. Zhao and S. Gao, CrystEngComm, 2012, 14, 5274 RSC
; (e) Z.-B. Zhu, W. Wan, Z.-P. Deng, Z.-Y. Ge, L.-H. Huo, H. Zhao and S. Gao, CrystEngComm, 2012, 14, 6675 RSC
.
- G. M. Sheldrick, SHELXTL-97, Program for Crystal Structure Solution and Refinement, University of Gottingen, Germany, 1997 Search PubMed
.
- D. Rendell, Fluorescence and Phosphorescence, Wiley, New York, 1987 Search PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Additional figures, PXRD patterns and TG curves. CCDC 1003905 and 1025993–1026004. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ra12338g |
| This journal is © The Royal Society of Chemistry 2014 |