Systematic theoretical investigation of geometries, stabilities and magnetic properties of iron oxide clusters (FeO)nμ (n = 1–8, μ = 0, ±1): insights and perspectives†
Abstract
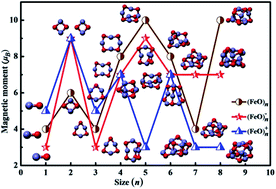
The structural properties of neutral and charged (FeO)nμ (n = 1–8, μ = 0, ±1) clusters have been studied using an unbiased CALYPSO structure searching method. As a first step, an unbiased search relying on several structurally different initial clusters has been undertaken. Subsequently, geometry optimization by means of density-functional theory with the Perdew and Wang (PW91) exchange–correlation functional is carried out to determine the relative stability of various candidates for low-lying neutral, anionic and cationic iron oxide clusters obtained from the unconstrained search. It is shown that the mostly equilibrium geometries of iron oxide clusters represent near planar structures for n ≤ 3. No significant structural differences are observed between the neutral and charged iron oxide clusters beyond sizes with n = 6. The relative stabilities of (FeO)nμ clusters for the ground-state structures are analyzed on the basis of binding energies and HOMO–LUMO gaps. Our theoretical results confirm that the binding energies of neutral and anionic (FeO)n0/− tend to increase with cluster size. Cationic (FeO)n+ exhibits a slight downward trend. It is worth noticing that (FeO)5 and (FeO)4−/+ are the most stable geometries for (FeO)nμ (n = 1–8, μ = 0, ±1) clusters. Lastly, an evident local oscillation of magnetic behavior is present in the most stable (FeO)nμ (n = 1–8, μ = 0, ±1) clusters, and the origin of this magnetic phenomenon is analyzed in detail.

 Please wait while we load your content...
Please wait while we load your content...