Water tuned nano/micro-structures in a redox-responsive supramolecular gel†
Yuxia Gaoa,
Jinrong Lua,
Jindan Wua,
Jun Hu*b and
Yong Ju*a
aKey Laboratory of Bioorganic Phosphorus Chemistry & Chemical Biology, Ministry of Education, Department of Chemistry, Tsinghua University, Beijing 100084, China. E-mail: juyong@tsinghua.edu.cn
bState Key Lab of Polymer Physics and Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, China. E-mail: jhu@ciac.ac.cn
First published on 17th November 2014
Abstract
A redox-responsive chiral supramolecular gel based on coumarin-tailed cholesterol linked with disulfide was reported. It was found that the gelation was primarily driven by the combination of hydrogen bonding, π–π stacking, and van der Waals forces. Moreover, its assembled morphology could be regulated from nanofibers to micro flowers and ribbons by water on account of its amphiphilic nature.
Introduction
Supramolecular gels, which utilize the self-assembly of organic molecules into entangled structures to immobilize the solvents, have attracted immense interest over the past decades for their potential applications in drug delivery, hybrid materials, oil recovery, and electronic devices.1 In the family of supramolecular gels, low-molecular-weight organogels (LMOGs) show higher superiority because of their accurate molecular weight, easy modification, multifarious nanostructures, and stimuli-responsive properties.2 Specifically, the stimuli-responsive gels are particularly appealing since their morphologies can be regulated easily in response to external stimuli, such as light, pH, ultrasound, ions, and oxidation/reduction.3 Although various assembled morphologies of gels have been reported, including fibers, ribbons, sheets, tubes, and flowers,4 the regulation of the morphologies is still a challenge. For example, Liu and co-workers reported that metal ions including Na+, Li+, Ni2+, Eu3+ and Tb3+ could turn the assembled nanostructure into a uniform helical twist in organogels.5a They also found that water could be used to tune the assembled nanostructures from nanofibers to helical tapes, helical tubes, and chiral nanotwists in organic solvents.5b Fang and co-workers discovered nanofibers and microballs could be obtained by changing the volume ratio of pyridine–methanol.6As a continuance of our previous works,7 herein we exploited a novel redox-responsive gelator 1 based on coumarin-tailed cholesterol linked with disulfide (Scheme 1) and its self-assembly behavior was investigated (Fig. 1). In such a molecule, coumarin, cholesterol, and disulfide are used as the sensor, gelation skeleton, and redox-responsive linker, respectively. It is known that disulfide can be reduced by dithiothreitol (DTT), glutathione (GSH) or PPh3 to sulfhydryl, which could be oxidized back to disulfide with the treatment of oxidants, such as oxygen, peroxide, and iodine.8 Therefore, disulfide has already been adopted in the self-healing materials, controlled release, and biosensor.9 Up to date, although many redox-responsive LMOGs based on ferrocene group,10 Cu(I)/Cu(II),3i or tetrathiafulvalene (TTF) group3j have been reported, the disulfide involved redox-responsive LMOGs are still rare.11
 | ||
| Fig. 1 Schematic illustration of the self-assembly process of the aggregates and the redox-responsive gel–sol transition triggered by DTT. The insets are photographs of gel and collapsed sol. | ||
Results and discussion
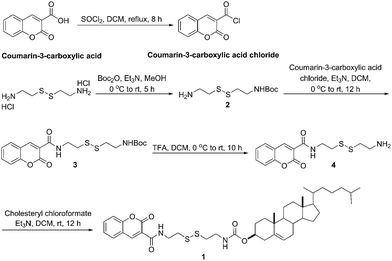
The gelator 1 was synthesized in multiple steps as shown in Scheme 1. Reaction between cystamine dihydrochloride and Boc anhydride afforded mono-protected amine 2,12 followed by the treatment with coumarin-3-carboxylic acid chloride to give 3. Subsequently 3 was deprotected by trifluoroacetic acid to give 4. Finally, the reaction between 4 and cholesteryl chloroformate was carried out in the presence of triethylamine to yield the coumarin–cholesterol conjugate 1, which was confirmed by 1H-, 13C NMR, ESI-MS, and HRMS (details see ESI†).As a general protocol, 1 was dissolved in various solvents, and tested by “stable to inversion of the test tube” method.7b,13 The results showed that 1 can form stable gel in DMF/H2O from the ratio of volume 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 5
1 to 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Table S1 and Fig. S1†). Its viscoelastic behaviour was characterized by the rheological measurements, in which the storage modulus G′ and the loss modulus G′′ were measured as functions of angle frequency and time sweep at 25 °C, respectively. As shown in Fig. 2a, G′ is around eight times greater than G′′, revealing the indispensable elastic behaviour of the gel.7c,14 The fact that G′ and G′′ decreased with the extension of time indicates that gel collapsed into the quasi-liquid state gradually (Fig. 2b).14a Moreover, the concentration-dependent sweep shows that G′ and G′′ ascended with the increase of gelator concentration (Fig. S2†).
1 (Table S1 and Fig. S1†). Its viscoelastic behaviour was characterized by the rheological measurements, in which the storage modulus G′ and the loss modulus G′′ were measured as functions of angle frequency and time sweep at 25 °C, respectively. As shown in Fig. 2a, G′ is around eight times greater than G′′, revealing the indispensable elastic behaviour of the gel.7c,14 The fact that G′ and G′′ decreased with the extension of time indicates that gel collapsed into the quasi-liquid state gradually (Fig. 2b).14a Moreover, the concentration-dependent sweep shows that G′ and G′′ ascended with the increase of gelator concentration (Fig. S2†).
Generally, the chirality of supramolecular systems can be generated through the assembly of chiral molecules.15 The CD spectra of 1 revealed a strong positive Cotton effect at 358 nm in gel state, while no signal was detected in solution (Fig. 2c). The signal intensity of the gel decreased gradually until disappeared completely with the temperature increasing from 20 to 50 °C (Fig. 2d). It indicates that the chirality is transformed from gelator 1 to the assembled aggregates during its chiral packing process.5,15
It is clearly seen that the gel turned opaque gradually with the increase of water (Fig. S1†). Studies on the thermal stability revealed that with the increase of water the minimum gelation concentration (MGC) decreased, while the gel-to-sol transition temperature (Tgel) increased (Fig. S3†). In order to investigate how the water tunes the gel morphologies, scanning electron microscopy (SEM), transmission electron microscopy (TEM) and atomic force microscopy (AFM) were performed. The results showed that a partial gel formed with the nanofibers ∼30–50 nm in diameter when water is less than 5% in DMF/H2O (Fig. 3a). Whereas a few micro-scale flower-like structures appeared co-existing with the nanofibers in the stable gel when the ratio of water reached 5% (Fig. 3b). The number of uniformed micro-scale flower-like architectures increased continuously with the increase of water in the mixed solvents (Fig. 3c). The flower-like architectures are ∼5–40 μm in diameter, and the thickness of the ‘flower petals’ (arrow in Fig. 3c) is ∼30–60 nm, which is close to fiber diameter. However, the further addition of water (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) led to collapse of the flowers, consequently generating micro-scale thick ribbons until all the flowers disappeared (Fig. 3d and e). When water was more than 20% in DMF/H2O, ribbons broke into fragments resulting in the collapse of gel (Fig. 3f). Moreover, the co-existing of nanofibers and micro-flowers in Fig. 3c can be further confirmed by TEM and AFM (Fig. 3g and h), in which both of them have the similar sizes as the ones under SEM. Apparently, the transformation from nanofibers to microflowers is a result of the anisotropic arrangement of molecules induced by the water content, consequently leading to the transformation between partial gel, stable gel, opaque gel, and collapsed gel.
1) led to collapse of the flowers, consequently generating micro-scale thick ribbons until all the flowers disappeared (Fig. 3d and e). When water was more than 20% in DMF/H2O, ribbons broke into fragments resulting in the collapse of gel (Fig. 3f). Moreover, the co-existing of nanofibers and micro-flowers in Fig. 3c can be further confirmed by TEM and AFM (Fig. 3g and h), in which both of them have the similar sizes as the ones under SEM. Apparently, the transformation from nanofibers to microflowers is a result of the anisotropic arrangement of molecules induced by the water content, consequently leading to the transformation between partial gel, stable gel, opaque gel, and collapsed gel.
To study the driving forces in the gelation process of 1, we investigated the temperature-dependent 1H NMR, IR, UV-Vis, and fluorescence spectroscopy. As the temperature increases, the protons of coumarin (H1, H2, H3, H4, H5) shifted upfield gradually as a result of the shielding effect, which strongly revealed the π–π stacking between the coumarin moieties (Fig. 4a), while no change was observed of the aliphatic protons (Fig. S4†). UV-Vis and fluorescence spectra provided more information about the π–π stacking. As shown in Fig. 4b, the absorption bands underwent a significant red shift from 295 to 300 nm with the increase of water content. Meanwhile, the addition of water to DMF solution of 1 led to a gradual decrease in the intensity at 413 nm and an appearance of a new band at 460 nm in fluorescence spectra (Fig. 4c). Apparently, the red shift ∼47 nm of emission band should be due to the excimer formation caused by π–π stacking between coumarin groups.14a Both red shifts in UV-Vis and fluorescence spectra strongly indicate the formation of “J”-type aggregates.16
Additionally, IR spectra of a powder sample and xerogel of 1 were compared (Fig. 4d). It showed that the stretching vibrations of N–H moiety and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O shifted from 3325, 1718 cm−1 to 3333, 1721 cm−1, respectively, upon the gelation. This result implied the formation of the intermolecular hydrogen bonding during the gelation process.4b Furthermore, the C–H stretching vibrations of alkyl groups appeared at a lower wavenumber (2936 cm−1) in the xerogel compared with that in the powder sample (2950 cm−1). It is known that the stretching vibrations of alkyl groups can be used as a sensitive sensor for the order of alkyl chains because of the association between the increase in frequencies and band widths with the increasing numbers of gauche defects and disorder.17 Therefore, the decrease in wavenumbers from the powder sample to xerogel revealed an increase of the van der Waals interactions among the neighboring alkyl chains. It is apparent that the intermolecular hydrogen bonding and van der Waals forces of alkyl chains play crucial roles in the formation of supramolecular gel besides the π–π stacking between the aromatic rings.
O shifted from 3325, 1718 cm−1 to 3333, 1721 cm−1, respectively, upon the gelation. This result implied the formation of the intermolecular hydrogen bonding during the gelation process.4b Furthermore, the C–H stretching vibrations of alkyl groups appeared at a lower wavenumber (2936 cm−1) in the xerogel compared with that in the powder sample (2950 cm−1). It is known that the stretching vibrations of alkyl groups can be used as a sensitive sensor for the order of alkyl chains because of the association between the increase in frequencies and band widths with the increasing numbers of gauche defects and disorder.17 Therefore, the decrease in wavenumbers from the powder sample to xerogel revealed an increase of the van der Waals interactions among the neighboring alkyl chains. It is apparent that the intermolecular hydrogen bonding and van der Waals forces of alkyl chains play crucial roles in the formation of supramolecular gel besides the π–π stacking between the aromatic rings.
X-ray diffraction (XRD) afforded the direct insight into the molecular packing pattern. As shown in Fig. 4e, the reflection peaks corresponding to d-spacing of 3.16, 1.58, 1.05, 0.79, 0.64 and 0.53 nm in the small angle region were observed, following the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/2
1/2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/3
1/3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/4
1/4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/5
1/5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/6. It strongly reveals the lamellar structure of the supramolecular gel. The fact that the interlayer distance (3.16 nm) is close to the extended molecular length of 1 (3.22 nm, Fig. 4e, inset) indicates that the “J”-type aggregates are formed by using the single molecule as a basic building block. Based on all the above results, it is apparent that the free molecule 1 in DMF is promoted to form ordered fibers upon the addition of water primarily driven by van der Waals forces, hydrogen bondings, and π–π stacking. These fibers can further entangle each other to form 3D nest-like networks to trap solvent molecules (Fig. 1, left). Whereas, on account of its amphiphilic nature, the fibers tend to arrange more closely to form flower-like aggregates bearing less hydrophobic areas with the increase of water in the system.
1/6. It strongly reveals the lamellar structure of the supramolecular gel. The fact that the interlayer distance (3.16 nm) is close to the extended molecular length of 1 (3.22 nm, Fig. 4e, inset) indicates that the “J”-type aggregates are formed by using the single molecule as a basic building block. Based on all the above results, it is apparent that the free molecule 1 in DMF is promoted to form ordered fibers upon the addition of water primarily driven by van der Waals forces, hydrogen bondings, and π–π stacking. These fibers can further entangle each other to form 3D nest-like networks to trap solvent molecules (Fig. 1, left). Whereas, on account of its amphiphilic nature, the fibers tend to arrange more closely to form flower-like aggregates bearing less hydrophobic areas with the increase of water in the system.
Due to the involvement of disulfide bond in the gelator, the supramolecular gel showed excellent redox-responsive to reductants. Upon the continuous addition of DTT solution (DMF/H2O) on the gel surface, the transparent gel gradually turned turbid until collapsed completely (Fig. 1, photography). After the cleavage of disulfide, fibers and flowers were destroyed to fragments as shown in SEM images (Fig. 1, right). The more direct evidence comes from ESI-MS analysis, in which the ion peak of compound 1 decreased notably companied with several new peaks corresponding to sulfhydryl compounds (Fig. S5†). For better understanding the role of disulfide in the redox-responsive process, the control molecule 5 (Fig. 5a, details see ESI†) was synthesized, in which the disulfide was replaced by the alkyl chain. Though 5 could also form gel in DMF/H2O (Fig. 5b), it didn't display any changes in the presence of DTT, highlighting the importance of disulfide bond to such a redox-responsive supramolecular gel.
 | ||
Fig. 5 (a) Molecular structure of compound 5; (b) SEM image of xerogel from 5 in DMF/H2O (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, 10 mg mL−1), scale bar is 10 μm. 1, v/v, 10 mg mL−1), scale bar is 10 μm. | ||
Conclusions
We have exploited a novel redox-responsive chiral supramolecular gel based on coumarin-tailed cholesterol linked with disulfide, primarily driven by the combination of hydrogen bonding, π–π stacking, and van der Waals forces. The gel morphology could be regulated by water from nanofibers to microflowers and microribbons on account of its amphiphilic characteristic. Moreover, such supramolecular gel exhibited excellent redox-responsive properties, which may be used in controlled release and drug delivery. Our results point out the potential self-assembled morphological control in area of supramolecular gel.Acknowledgements
This work was supported by NNSF of China (no. 21172130, 21472108) and NBRP of China (no. 2012CB8210601). JH is thankful for the support of State Key Laboratory of Polymer Physics and Chemistry, CIAC, China.Notes and references
- (a) N. M. Sangeetha and U. Maitra, Chem. Soc. Rev., 2005, 34, 821 RSC; (b) A. Ajayaghosh, V. K. Praveen and C. Vijayakumar, Chem. Soc. Rev., 2008, 37, 109 RSC; (c) S. R. jadhav, P. K. Vemula, R. Kumar, S. R. Raghavan and G. John, Angew. Chem., Int. Ed., 2010, 49, 7695 CrossRef CAS PubMed; (d) A. R. Hirst, B. Escuder, J. F. Mairavet and D. K. Smith, Angew. Chem., Int. Ed., 2008, 47, 8002 CrossRef CAS PubMed; (e) A. Friggeri, B. L. Feringa and J. van Esch, J. Controlled Release, 2004, 97, 241 CrossRef CAS PubMed; (f) M. Cametti and Z. Džolić, Chem. Commun., 2014, 50, 8273 RSC; (g) J. W. Steed, Chem. Commun., 2011, 47, 1379 RSC.
- (a) P. Terech and R. G. Weiss, Chem. Rev., 1997, 97, 3133 CrossRef CAS PubMed; (b) M. Geroge and R. G. Weiss, Acc. Chem. Res., 2006, 39, 489 CrossRef PubMed; (c) X. Y. Hou, D. Gao, J. L. Yan, Y. Ma, K. Q. Liu and Y. Fang, Langmuir, 2011, 27, 12156 CrossRef CAS PubMed; (d) J. Raeburn, A. Z. Cardoso and D. J. Adams, Chem. Soc. Rev., 2013, 42, 5143 RSC; (e) B. G. Bag and S. S. Dash, Nanoscale, 2011, 3, 4564 RSC; (f) B. G. Bag and K. Paul, Asian J. Org. Chem., 2012, 1, 150 CrossRef CAS; (g) B. G. Bag and R. Majumdar, RSC Adv., 2012, 2, 8623 RSC.
- (a) M. D. Segarra-Maset, V. J. Nebot, J. F. Miravet and B. Escuder, Chem. Soc. Rev., 2013, 42, 7086 RSC; (b) X. D. Yu, L. M. Chen, M. M. Zhang and T. Yi, Chem. Soc. Rev., 2014, 43, 5346 RSC; (c) K. Murata, M. Aoki, T. Suzuki, T. Harada, H. Kawabata, T. Komori, F. Ohseto, K. Ueda and S. Shinkai, J. Am. Chem. Soc., 1994, 116, 6664 CrossRef CAS; (d) S. Sumiya, Y. Shiraishi and T. Hirai, New J. Chem., 2013, 37, 2642 RSC; (e) C. Y. Zhou, W. X. Gao, K. W. Yang, L. Xu, J. C. Ding, J. X. Chen, M. C. Liu, X. B. Huang, S. Wang and H. Y. Wu, Langmuir, 2013, 29, 13568 CrossRef CAS PubMed; (f) X. D. Yu, Q. Liu, J. C. Wu, M. M. Zhang, X. H. Gao, S. Zhang, Q. Wang, L. M. Chen and T. Yi, Chem.–Eur. J., 2010, 16, 9099 CrossRef CAS PubMed; (g) X. D. Yu, Y. J. Li, Y. B. Yin and D. C. Yu, Mater. Sci. Eng., C, 2012, 32, 1695 CrossRef CAS PubMed; (h) K. Ghosh and D. Kar, Org. Biomol. Chem., 2012, 10, 8800 RSC; (i) S. Kawano, N. Fujita and S. Shinkai, J. Am. Chem. Soc., 2004, 126, 8592 CrossRef CAS PubMed; (j) C. Wang, D. Q. Zhang and D. B. Zhu, J. Am. Chem. Soc., 2005, 127, 16372 CrossRef CAS PubMed.
- (a) A. Vidyasagar, K. Handore and K. M. Sureshan, Angew. Chem., 2011, 123, 8171 CrossRef; (b) Y. F. Zhou, T. Yi, T. C. Li, Z. G. Zhou, F. Y. Li, W. Huang and C. H. Huang, Chem. Mater., 2006, 18, 2974 CrossRef CAS; (c) P. Y. Xing, X. X. Chu, M. F. Ma, S. Y. Li and A. Y. Hao, Phys. Chem. Chem. Phys., 2014, 16, 8346 RSC; (d) V. Lozano, R. Hernandez, C. Mijangos and M. Pérez-Pérez, Org. Biomol. Chem., 2009, 7, 364 RSC; (e) S. Ghosh and S. Verma, Tetrahedron, 2008, 64, 6202 CrossRef CAS PubMed; (f) A. Ajayaghosh, R. Varghese, V. K. Praveen and S. Mahesh, Angew. Chem., Int. Ed., 2006, 45, 3261 CrossRef CAS PubMed; (g) N. S. S. Kumar, S. Varghese, G. Narayan and S. Das, Angew. Chem., 2006, 118, 6465 CrossRef; (h) G. S. Lim, B. M. Jung, S. J. Lee, H. H. Song, C. Kim and J. Y. Chang, Chem. Mater., 2007, 19, 460 CrossRef CAS.
- (a) X. F. Wang, P. F. Duan and M. H. Liu, Chem. Commun., 2012, 48, 7501 RSC; (b) C. X. Liu, Q. X. Jin, K. Lv, L. Zhang and M. H. Liu, Chem. Commun., 2014, 50, 3702 RSC.
- Z. Y. Xu, J. X. Peng, N. Yan, S. S. Zhang, K. Q. Liu and Y. Fang, Soft Matter, 2013, 9, 1091 RSC.
- (a) J. Hu, M. Zhang and Y. Ju, Soft Matter, 2009, 5, 4971 RSC; (b) J. R. Lu, J. Hu, Y. Song and Y. Ju, Org. Lett., 2011, 13, 3372 CrossRef CAS PubMed; (c) J. R. Lu, J. Hu, C. L. Liu, H. X. Gao and Y. Ju, Soft Matter, 2012, 8, 9576 RSC; (d) J. R. Lu, Y. X. Gao, J. D. Wu and Y. Ju, RSC Adv., 2013, 3, 23548 RSC; (e) J. Hu, J. D. Wu, Q. Wang and Y. Ju, Beilstein J. Org. Chem., 2013, 9, 2877 CrossRef PubMed; (f) J. R. Lu, J. D. Wu and Y. Ju, New J. Chem., 2014, 38, 6050 RSC.
- (a) V. Haridas, S. Sahu and A. R. Sapala, Chem. Commun., 2012, 48, 3821 RSC; (b) Z. G. Tao, Z. Y. Xiao, X. Zhao, X. K. Jiang and Z. T. Li, Tetrahedron Lett., 2012, 53, 4447 CrossRef CAS PubMed.
- (a) B. Khorsand, G. Lapointe, C. Brett and J. K. Oh, Biomacromolecules, 2013, 14, 2103 CrossRef CAS PubMed; (b) J. Canadell, H. Goossens and B. Klumperman, Macromolecules, 2011, 44, 2536 CrossRef CAS; (c) G. H. Deng, F. Y. Li, H. X. Yu, F. Y. Liu, C. Y. Liu, W. X. Sun, H. F. Jiang and Y. M. Chen, ACS Macro Lett., 2012, 1, 275 CrossRef CAS; (d) R. Liu, X. Zhao, T. Wu and P. Y. Feng, J. Am. Chem. Soc., 2008, 130, 14418 CrossRef CAS PubMed; (e) R. Liu, Y. Zhang and P. Y. Feng, J. Am. Chem. Soc., 2009, 131, 15128 CrossRef CAS PubMed; (f) M. H. Lee, Z. G. Yang, C. W. Lim, Y. H. Lee, S. Dongbang, C. Kang and J. S. Kim, Chem. Rev., 2013, 113, 5071 CrossRef CAS PubMed; (g) M. M. Pires and J. Chmielewski, Org. Lett., 2008, 10, 837 CrossRef CAS PubMed; (h) M. H. Lee, J. H. Han, P. Kwon, S. Bhuniya, J. Y. Kim, J. L. Sessler, C. Kang and J. S. Kim, J. Am. Chem. Soc., 2012, 134, 1316 CrossRef CAS PubMed; (i) J. Ryu, S. Park, B. Kim, A. Klaikherd, T. P. Russell and S. Thayumanavan, J. Am. Chem. Soc., 2009, 131, 9870 CrossRef CAS PubMed.
- (a) J. Liu, J. L. Yan, X. W. Yuan, K. Q. Liu, J. X. Peng and Y. Fang, J. Colloid Interface Sci., 2008, 318, 397 CrossRef CAS PubMed; (b) P. L. He, J. Liu, K. Q. Liu, L. P. Ding, J. L. Yan, D. Gao and Y. Fang, Colloids Surf., A, 2010, 362, 127 CrossRef CAS PubMed; (c) R. Afrasiabi and H. Kraatz, Chem.–Eur. J., 2013, 19, 17296 CrossRef CAS PubMed; (d) B. Adhikari and H. Kraatz, Chem. Commun., 2014, 50, 5551 RSC; (e) B. Adhikari, R. Afrasiabi and H. Kraatz, Organometallics, 2013, 32, 5899 CrossRef CAS.
- (a) J. Chen, W. W. Wu and A. J. McNeil, Chem. Commun., 2012, 48, 7310 RSC; (b) C. H. Ren, Z. J. Song, W. T. Zheng, X. M. Chen, L. Wang, D. L. Kong and Z. M. Yang, Chem. Commun., 2011, 47, 1619 RSC.
- J. Niu, Z. H. Liu, L. Fu, F. Shi, H. W. Ma, Y. Ozaki and X. Zhang, Langmuir, 2008, 24, 11988 CrossRef CAS PubMed.
- D. G. Velázquez, D. D. Díaz, Á. G. Ravelo and J. J. Marrero-Tellado, Eur. J. Org. Chem., 2007, 11, 1841 CrossRef.
- (a) M. Dubey, A. Kumar and D. S. Pandey, Chem. Commun., 2014, 50, 1675 RSC; (b) M. Dubey, A. Kumar, R. K. Gupta and D. S. Pandey, Chem. Commun., 2014, 50, 8144 RSC; (c) H. B. Wei, N. Shi, J. L. Zhang, Y. Guan, J. Zhang and X. H. Wan, Chem. Commun., 2014, 50, 9333 RSC.
- (a) D. K. Smith, Chem. Soc. Rev., 2009, 38, 684 RSC; (b) P. F. Duan, H. Cao, L. Zhang and M. H. Liu, Soft Matter, 2014, 10, 5428 RSC; (c) H. Cao, X. F. Zhu and M. H. Liu, Angew. Chem., Int. Ed., 2013, 52, 4122 CrossRef CAS PubMed; (d) X. F. Wang, F. F. Duan and M. H. Liu, Chem.–Asian J., 2014, 9, 770 CrossRef CAS PubMed.
- (a) S. Abraham, R. K. Vijayaraghavan and S. Das, Langmuir, 2009, 25, 8507 CrossRef CAS PubMed; (b) A. Ajayaghosh, C. Vijayakumar, R. Varghese and S. J. George, Angew. Chem., Int. Ed., 2006, 45, 456 CrossRef CAS PubMed; (c) K. Jang, L. V. Brownell, P. M. Forster and D. C. Lee, Langmuir, 2011, 27, 14615 CrossRef CAS PubMed; (d) H. Yao, T. Isohashi and K. Kimura, J. Phys. Chem. B, 2007, 111, 7176 CrossRef CAS PubMed.
- (a) M. J. Hostetler, J. J. Stokes and R. W. Murray, Langmuir, 1996, 12, 3604 CrossRef CAS; (b) N. Yamada, K. Ariga, M. Naito, K. Matsubara and E. Koyama, J. Am. Chem. Soc., 1998, 120, 12192 CrossRef CAS; (c) S. E. Paramonov, H.-W. Jun and J. D. Hartgerink, J. Am. Chem. Soc., 2006, 128, 7291 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis and structure characterization of compounds 1 and 5; gelation behaviour of 1 in various solvents; plots of Tgel against the concentration of 1; temperature-dependent 1H NMR of 1; concentration-dependent rheological measurements of the gel; SEM images of 5; ESI-MS spectrum of the gel 1 after the DTT addition. See DOI: 10.1039/c4ra12247j. |
| This journal is © The Royal Society of Chemistry 2014 |