Retracted Article: Investigation of anticorrosive, antibacterial and in vitro biological properties of a sulphonated poly(etheretherketone)/strontium, cerium co-substituted hydroxyapatite composite coating developed on surface treated surgical grade stainless steel for orthopedic applications
Abstract
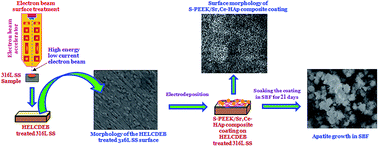
In the present investigation, a sulphonated poly(etheretherketone)/strontium, cerium co-substituted hydroxyapatite (S-PEEK/Sr,Ce-HAp) composite coating is obtained on high energy low current DC electron beam (HELCDEB) treated 316L stainless steel (316L SS) by electrodeposition. The surface of the 316L SS was treated using HELCDEB with an energy of 500 keV and a beam current of 1.5 mA. The as-formed coatings on HELCDEB treated 316L SS were characterised by X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FT-IR) and high resolution scanning electron microscopy (HRSEM). Electrochemical results show that the S-PEEK/Sr,Ce-HAp coating with an optimum 2 wt% S-PEEK concentration on HELCDEB treated 316L SS possesses maximum corrosion resistance in Ringer’s solution. The antibacterial activity and in vitro bioactivity of the composite coatings were investigated. The results revealed that the HELCDEB treatment of the 316L SS improved anticorrosion performance and also that the combination of S-PEEK and Sr,Ce-HAp in the coating greatly improved the bioactivity and biocompatibility of the as-developed composite coating on HELCDEB treated 316L SS.

 Please wait while we load your content...
Please wait while we load your content...