Porous cerium dioxide hollow spheres and their photocatalytic performance†
Saisai Yuanab,
Qitao Zhangab,
Bin Xuabc,
Zhengyuan Jina,
Ya Zhange,
Yin Yanga,
Ming Zhang*bc and
Teruhisa Ohno*ad
aDepartment of Applied Chemistry, Faculty of Engineering, Kyushu Institute of Technology, Kitakyushu 804-8550, Japan. E-mail: tohno@che.kyutech.ac.jp; Fax: +81 93 884 3318; Tel: +81 93 884 3318
bSchool of Chemistry and Chemical Engineering, Yangzhou University, Yangzhou 225002, China. E-mail: lxyzhangm@yzu.edu.cn; Fax: +86 51487979244; Tel: +86 51487990926
cTest Center, Yangzhou University, Yangzhou 225002, China
dJST, PRESTO and ACT-C, 4-1-8 Honcho, Kawaguchi, Saitama 332-0012, Japan
eSchool of Environmental Science and Engineering, Yangzhou University, Yangzhou 225002, China
First published on 12th November 2014
Abstract
Uniform-sized and monodiperse cerium dioxide porous hollow spheres (CeO2-PH) based on the Ostwald ripening process were fabricated by a simple solvothermal method in the absence of any templates. The structure and morphology of CeO2-PH and CeO2-NP (cerium dioxide nanoparticles) were characterized by X-ray diffraction (XRD), transmission electron microscopy (TEM), field emission scanning electron microscopy (FESEM), and Brunauer–Emmett–Teller (BET) surface area analysis. The average diameter of face-centered cubic (fcc) phase CeO2-PH was ca. 160 nm with a high specific surface area, and it is composed of small crystal grain particles (ca. 10 nm). Furthermore, CeO2-PH has high activity for the evaluation of acetaldehyde decomposition. Optical, defect, and chemical state properties were characterized by Raman spectra, ultraviolet-visible absorption spectroscopy (UV-vis), and X-ray photoelectron spectroscopy (XPS). The presence of Ce3+ ions narrowed the band gap of CeO2-PH, resulting in the high light harvesting. The large amount of oxygen vacancy defects provided many activity sites on CeO2-PH in the photocatalytic process. The formation scheme and photocatalyic mechanism will be discussed in this paper.
Introduction
Nanomaterials with a hollow structure have attracted considerable interest in the past few decades due to their low density, high surface area, and wide range of applications in areas such as catalysis, drug delivery, and gas storage.1–4 Recent developments in the synthesis of particles with a hollow structure have enabled their properties including their mechanical, optical, electrical and chemical properties to be tuned to some degree. For instance, Ma et al. have recently modified a previously reported method for Si nanoparticle synthesis to prepare nest-like Si hollow nanospheres with great improvements in cycle life and rate capability.5 α-Fe2O3 hollow nanospheres, for example, have been shown to have improved photocatalytic performance over α-Fe2O3 nanocrystals in oxidation of salicylic acid.6 Hollow materials with a porous structure have received much more attention because of their novel properties and potential applications. A porous-hollow structure has a larger surface area, and strong adsorption capacity. This special structure may provide new options for the removal of organic pollutants from waste water.7Cerium oxide is one of the most important earth metal oxides, which has been extensively used in various applications including applications in UV blockers, polishing materials, catalysts, electrolytes, sensors, and solar cells due to its favorable properties including chemical stability, redox property, and high oxygen storage capacity.2,8,9 Notably, ceria is a vital component in three-way catalysts (TWCs) mainly due to its high degree of tolerance to reversible oxygenation–deoxygenation cycles without disruption of fluorite lattice structure.10–12 In the past few decades, various morphologies of ceria including cubes, octshedra, spheres, wires and rods have been investigated. Very recently, ceria with hollow a structure has been fabricated by various methods.13–16 Thin layers (ca. 12 nm) of cerium oxide were deposited onto ca. 200 nm-thick silica colloid templates using cerium nitrate and the silica cores were subsequently removed to yield hollow spheres.17 Novel single-crystalline-like CeO2 hollow nanocubes were synthesized through a solvothermal method using peroxyacetic acid (PAA) as the oxidant in the absence of a template. Solid evacuation in the central part via Ostwald ripening led to the formation of single-crystalline-like hollow nanocubes.18 Liang et al. synthesized CeO2–ZrO2 solid solution nanocages with controllable structures via the Kirkendall effect.19 Different morphologies would have various application, especially, the hollow structure was favourable for the catalysis.
Literature reported some special morphologies of ceria, which were applied to the photocatalysis.20,21 In this work, ceria porous-hollow spheres were synthesized by a simple solvothermal method in the absence of any templates via Ostwald ripening. The morphology, structure and other properties were characterized by TEM, FESEM, BET, XRD, Raman, UV-vis, and XPS, and the photocatalyic performance was evaluated by degradation of acetaldehyde. Compared with the previous reports, the synthetic process was one-step and very simple, simultaneously, the special porous hollow spheres structure was applied to the degradation of acetaldehyde, which had many potential applications.
Experimental section
All chemicals used in the experimental section were analytical grade and used as received without further purification.Photocatalyst preparation
CeO2 hollow spheres with ∼140 nm in diameters were synthesized by a simple one-step template-free solvothermal method with reaction in a mixed solution of water, ethanol and glycol together with PVP (polyvinyl pyrrolidone) as a surfactant. Typically, 1.0 g of Cerium(III) nitrate hexahydrate and 0.10 g of PVP were dissolved in a mixture of EG (20 mL), EtOH (40 mL) and H2O (20 mL) with magnetic stirring for 3 h. Then the suspension was transferred to a 100 mL Teflon-lined autoclave and heated at 180 °C for 24 h. After cooling to room temperature, the product was collected by centrifugation, washed with ethyl alcohol and water until the ionic strength was less than 10 μs cm−1, and dried at 70 °C overnight. Finally, we obtained hollow-sphere cerium dioxide, which was denoted as CeO2-PH.For control experiments, ceria nanoparticles were obtained under same conditions as those described above but in the absence of glycol, and the nanoparticles were denoted as CeO2-NP.
Characterization
The CeO2 materials were characterized using X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), field emission scanning electron microscopy (FESEM), transmission electron microscopy (TEM), high-resolution (HRTEM), ultraviolet-visible absorption spectroscopy (UV-vis), Brunauer–Emmett–Teller (BET) surface area analysis and Raman spectra.The crystal structure and composition were determined by XRD using a Rigaku MiniFlex II X-ray diffractometer with a Cu-Kα radiation source (γ = 1.5405 Å). The morphologies of the samples were observed by FESEM (JEOL, JSM-6701FONO) and TEM (Hitachi, H-9000NAR, 200 kV). HRTEM analysis was conducted using a Tecnai G2 F30 S-TWIN (300 kV). Nitrogen adsorption/desorption measurements were performed at 77 K using a Quantachrome Nova 4200e to calculate the specific surface area using the BET model. The pore size distribution was obtained from desorption-isotherm curves by the Barrett–Joyner–Halenda (BJH) method. Prior to measurements, the samples were degassed in vacuum at 180 °C for 3 h. Diffuse reflectance (DR) spectra were measured using a UV-vis spectrophotometer (Shimadzu, UV-2500PC) equipped with an integrating sphere unit (Shimadzu, ISR-240A). Raman spectra were obtained by a laser Raman spectrum (JASCO, NRS-5100). An XPS experiment was carried on a Thermo ESCALAB 250Xi system at room temperature under Al Kα using monochromatic radiation and C1s peak (284.70 ± 0.1 eV) reference. The background of XPS spectra was subtracted by the Shirley procedure and the peaks were fitted using the Gaussian–Lorentzian function.
Photocatalytic activity test
Photocatalytic activity of the samples was evaluated by photocatalytic decomposition of acetaldehyde in gas phase. Samples powder (150 mg), which had completed extinction of incident radiation, was spread on a glass dish, and the glass dish was put into a Tedlar bag (AS ONE Co. Ltd.) with a volume of 125 mL mixed air (79% N2, 21% O2, <0.1 ppm of CO2, 500 ppm of acetaldehyde). After 2 h adsorption equilibrium in the dark, the photocatalysts were exposed under the visible light. A light-emitting diode (LED; Lumileds, Luxeon LXHL-NRR8), which emitted light at a wavelength of ca. 435 nm with an intensity of 3.0 mW cm−2, was used as visible-light irradiation source. In the photocatalysis process, generation of carbon dioxide and consumption of acetaldehyde were monitored by online gas chromatography (Agilent Technologies, 3000A Micro-GC, TCD detector) equipped with OV1 and PLOT-Q columns.Results and discussion
The phase purity of the prepared products was estimated by the XRD patterns. Fig. 1 Shows the XRD patterns of CeO2-NP and CeO2-PH. All of the characteristic peaks were indexed to the face-centered cubic phase with space group Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m of ceria (JCPDS card no. 34-0394). No other peaks were detected, and the sharp peaks indicated that the high purity and the highly crystalline nature of both samples. Compared with the CeO2-NP, the peaks of CeO2-PH were relatively broad, demonstrating that the CeO2-PH was composed of smaller crystals. The average primary particle size was ca. 10 nm (Table 1), which is according to the calculation with the Debye–Scherrer formula for the strongest peak (111).
m of ceria (JCPDS card no. 34-0394). No other peaks were detected, and the sharp peaks indicated that the high purity and the highly crystalline nature of both samples. Compared with the CeO2-NP, the peaks of CeO2-PH were relatively broad, demonstrating that the CeO2-PH was composed of smaller crystals. The average primary particle size was ca. 10 nm (Table 1), which is according to the calculation with the Debye–Scherrer formula for the strongest peak (111).
 | ||
| Fig. 1 XRD patterns of the CeO2 nanoparticles (CeO2-NP), and the CeO2 porous-hollow spheres (CeO2-PH). | ||
| Type | Average primary particle size (Å) | SBET (m2 g−1) | Pore size distribution (nm)/type | Pore volume (cm3 g−1) |
|---|---|---|---|---|
| CeO2-PH | 103 | 65.91 | 3–30/mesoporous | 0.181 |
| CeO2-NP | 298 | 20.08 | 10–350/macroporous | 0.173 |
The morphologies of the as-prepared CeO2 photocatalysts were shown in Fig. 2. Fig. 2A shown an SEM image of ceria hollow spheres, which were composed of small nanoparticles. The diameter of the ceria hollow spheres was ca. 160 nm, and the particle size of the small nanoparticles was ca. 10 nm, which was in accordance with the XRD calculation (Fig. 1). The voids that can be seen in Fig. 2A suggested that the obtained ceria are porous-hollow spheres. TEM and HRTEM were performed to obtain more information about the special morphology. The low TEM image in Fig. 2B clearly shown a hollow structure of the prepared sample. It had a narrow size distribution and the diameter was in accordance with the SEM observation. In the amplified TEM image shown in Fig. 2C, the contrast between the dark margins and the pale center confirmed the existence of a hollow structure. Moreover, it revealed that the hollow ceria spheres consisted of small nanoparticles with a porous structure. This special morphology with a high specific surface area would be favorable for a photocatalysis process due to the full access of reactants (acetaldehyde in this study). The structure of the as-obtained ceria porous-hollow sphere nanocrystals was investigated in more detail by HRTEM (Fig. 2C inset). The spacing of the measured 2D lattice fringes was close to 0.27 nm indexed to the interplanar spacing of the (100) plane of the outside and inside surfaces.22–24 To understand the formation mechanism of the porous-hollow structure, samples synthesized with different reaction times were collected and analyzed. A plausible formation scheme of the hollow CeO2 sphere is illustrated in Scheme 1. When PVP and Ce(NO3)3·6H2O are dissolved in an aqueous ethanol and EG solution resulting in a homogeneous mixture, cerium ions are well surrounded by PVP molecules due to the strong interaction between the nuclei and surfactant. Nanoparticles followed by self-assembling of ceria nanoparticles to form a sphere shape because of the isotropic growth. The hollow shape is formed because cerium nanoparticles tend to move towards the wall of the sphere due to the density variation among nanoparticles and then undergo the Ostwald ripening process.7,25,26 Due to the difference of surface energy and particles located in the inner space of the spheres and this particles could be dissolved and merged by particles in the outer surface, and meanwhile the solid sphere gradually develops into a hollow structures. The factors of the porous-hollow structure formation will be presented in ESI (Fig. S1†). Fig. 2D–F show SEM, TEM, and HRTEM images of the ceria nanoparticles, which was set as the contrast samples. The sizes of nanoparticles were about 50–100 nm, and slight agglomeration can be seen in the SEM image in Fig. 2D of the SEM image. Compared to previously prepared ceria, these ceria nanoparticles had better crystallinity as shown in the HRTEM image in Fig. 2F, though the dispersibility was not so good due to the absence of glycol. Glycol possesses a capping reagent function, and it also has a possibility of dissolution of the metal salt. The lattice fringe in the HRTEM image (Fig. 2F inset) show a spacing of 0.31 nm from the (111) plane of the cubic ceria.22
 | ||
| Fig. 2 SEM images of (A) the CeO2 hollow spheres, (D) the CeO2 nanoparticles; TEM images of (B) & (C) the CeO2 hollow spheres, (E) & (F) the CeO2 nanoparticles, the inset is the HRTEM images. | ||
 | ||
| Scheme 1 Formation of the porous-hollow structure; (A)–(D) are corresponding to the Fig. S1.† | ||
Furthermore, the N2 adsorption–desorption isotherms and pore size distribution of CeO2-PH and CeO2-NP are shown in Fig. 3. The shape of the isotherm (Fig. 3a) with a hysteresis loop ranging from 0.4 to 1.0 in the relative pressure corresponds to a type-IV isotherm according to the Brunauer–Deming–Deming–Teller (BDDT) classification, simultaneously revealing the existence of a mesoporous structure in CeO2-PH. Fig. 3b (CeO2-NP) exhibits the type-III isotherms with hysteresis loops at the relative pressures of 0.8–1.0, indicating the presence of disordered macroporous structure.27 The pore size distribution of CeO2-PH (Fig. 3a inset) was determined by the Barrett–Joyner–Halenda (BJH) method from the desorption branch of the isotherm. The pore size distribution is narrow, from 3 nm to 30 nm, in the mesoporous region, centered at 9 nm, and the pore volume is 0.181 cm g−1, which is attributed to the aggregation of small crystal particles. However, CeO2-NP (Fig. 3b inset) has a broad pore size distribution ranging from 10 nm to 350 nm and the pore volume is 0.173 cm g−1, which is attributed to the void spaces among the stacked ceria nanoparticles. The specific surface areas of the CeO2-PH and CeO2-NP were 65.91 m2 g−1, and 20.08 m2 g−1 respectively, which were calculated by the Brunauer–Emmett–Teller (BET) equation (Table 1).
 | ||
| Fig. 3 N2 adsorption–desorption isotherm, and inset is the corresponding BJH pore size distribution curves, (a) the ceria porous-hollow spheres, (b) the ceria nanoparticles. | ||
Table 1 is a summary of the results of XRD and BET calculations results. Compared with CeO2-NP, CeO2-NP has a small average primary particle size, high specific surface area, narrow pore size distribution and large pore volume, which are attributed to the smaller crystal size and the special porous-hollow sphere structure. These excellent properties will be favourable for a photocatalysis process.
Photocatalytic activity study
The photocatalytic activity of CeO2-PH was examined in acetaldehyde decomposition reaction under visible light irradiation, and the activity of CeO2-NP and P25 were also estimated in the same condition for comparison. Fig. 4a shows the CO2 evaluation activities of CeO2-NP, CeO2-PH and P25. After 24 h visible light irradiation, the degradation efficiencies of P25, CeO2-NP and CeO2-PH were ca. 5%, 25% and 92%, respectively. The activity of CeO2-PH sample was about 4-times higher than the contrast sample for CeO2-NP, and the 18-times higher than the P25 (P25 has no response to the visible light). One plausible reason of the higher activity is the larger SBET of CeO2-PH. Compared with CeO2-NP the porous and hollow structure was the predominant reason for the higher SBET, which provided superior adsorption and reactive sites for decomposition of acetaldehyde. However, the primary particle size (ca. 10 nm) of the CeO2-PH was smaller than that of CeO2-NP (50–100 nm), as observed in FESEM images. The presence of quantum effects has a great influence on properties of the surfaces of samples, such as electrical and optical performances. These special properties play the critical roles in the photocatalysis process, which can be as another more important credible reason for the excellent photocatalytic performance. The surface property and mechanism are discussed in detail in the following section. In order to estimate the stability of the CeO2-PH, cycling performance was tested. The result was shown in Fig. 4b. After 6 h irradiation, the generation of CO2 were 376.226 ppm (first), 361.593 ppm (second) and 370.159 ppm (third), which indicated that the stability of photocatalyst (CeO2-PH) was excellent. | ||
| Fig. 4 (a) Time courses of CO2 evolution from acetaldehyde decomposition over the products under visible-light irradiation, (b) cycling performance of CeO2-PH. | ||
For ultraviolet-blocking materials, ceria has a strong absorption in the ultraviolet range. Fig. 5 shows UV-vis diffuse reflectance spectra of CeO2-NP and CeO2-PH. There is a strong absorption band from 300 nm to 350 nm in the spectra, which is assigned to the charge transfer from O2− in O 2p to Ce4+ in Ce 4f. The prepared CeO2-PH had fractional absorption in the visible region. As semiconductor materials, the direct band gap (Eg) can be calculated from the equation of αhν = A(hν − Eg), where hν is the photon energy, α is the absorption coefficient, and A is a constant of CeO2.28 Calculated Eg values for CeO2-NP and CeO2-PH were 2.88 eV and 3.01 eV, respectively (Fig. 5 inset), which are smaller than the theoretical value of 3.2 eV for bulk CeO2. There are two plausible theories for the expatiation of the final moderato red-shift of the CeO2-PH. The existence of quantum confinement effect due to the nanoscale size of the primary particles forming the porous-hollow spheres resulted in a blue-shift in the UV-vis diffuse reflectance spectrum. Simultaneously, the decrease in primary particle size led to an increase in the Ce3+ ion concentration29 (XPS results in Fig. 7). Chen et al. found out that the blue-shift of the absorption edge in a CeO2 film occurred with a decrease in the Ce3+ content.30,31 Therefore, the red-shift of the band gap for CeO2-PH should originate from the transformation between Ce4+ to Ce3+.32 In conclusion, band gap narrowing is the integrated result of the two mentioned reasons, which is beneficial for the photocatalytic process.
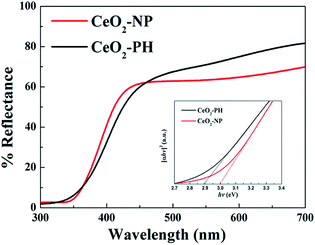 | ||
| Fig. 5 UV-vis diffuse reflectance spectra of CeO2-NP and CeO2-PH; the inset is the (αhν)2 versus hν plots curves. | ||
 | ||
| Fig. 6 Raman spectra of samples (a) the CeO2-PH, (b) the CeO2-NP, (c) enlarged image on dominant peak of both CeO2-PH and CeO2-NP. | ||
The visible Raman spectra (Fig. 6) are dominated by a strong F2g symmetry mode of CeO2 fluorite phase at 464 cm−1 on CeO2 with weak bands at 258 cm−1 and 595 cm−1, due to second-order transverse acoustic (2TA) mode and defect-induced (D), respectively. The slight shift to 461 cm−1 (Fig. 6c inset) and the obvious lower intensity of this mode implied that CeO2-PH had a stronger the symmetry of Ce–O bond also led to a stronger optical absorption as different colors on these samples (grey on CeO2-NP and yellow on CeO2-PH, in accordance with the UV-vis test).33 The ionic radii of Ce3+ and Ce4+ are 1.034 Å and 0.92 Å, respectively. When Ce3+ (Fig. 7 XPS results) was induced Ce–O bond, the lattice constant increased, sequentially, slight red-shift occurred.34,35 Compared with CeO2-NP (Fig. 6b), CeO2-PH (Fig. 6a) shown a stronger intensity in ca. 595 cm−1 (D), indicating that CeO2-PH had much more intrinsic defects. The relative intensity ratios of ID/IF2g were calculated to be about 1.8% (CeO2-PH) and 0.5% (CeO2-NP). It has been proved that the presence of surface defects, such as large size oxygen vacancy clusters,36 would promote the transformation of Ce4+ to Ce3+ for CeO2-based materials.
In order to investigate in more detail the surface composition and chemical state, XPS analysis was carried out. The CeO2 spectrum is composed of two multiplets (v and u), which correspond to the spin–orbit split 3d5/2 and 3d3/2 core holes. Fig. 7 shows the Ce 3d3/2 and Ce 3d5/2 spectra of the CeO2-PH (a) and CeO2-NP (b), respectively. The spectra of Ce 3d can be decomposed into ten peaks by Gaussian–Lorentzian function fitting. According to previous research, the labels u, u′′, u′′′, v, v′′ and v′′′ refer to Ce 3d3/2 and Ce 3d5/2 are characteristic peaks of Ce4+ in CeO2. The highest binding energy (BE) peaks U′′′ and V′′′ are located at 916.9 eV and 898.2 eV and arise from the Ce (3d94f0) O (2p6) final state. The lower BE states U′′ and V′′ are located at 907.5 eV and 888.95 eV and are assigned to Ce (3d94f1) O (2p5). The BE peaks of U and V at 901.1 eV and 881.89 eV are attributed to the Ce (3d94f2) O (2p4) final state. In case of Ce 3d of Ce3+, BE peaks of Ce 3d consist of two pairs of doublets (U0, V0, U′ and V′). For Ce3+, the highest BE peaks U′ and V′ appear at 903.4 eV and 885.02 eV, respectively. These doublets correspond to Ce (3d94f1) O (2p5). The lowest BE peaks U0 and V0 appear at 880.2 eV and 898.2 eV and correspond to Ce (3d94f1) O (2p6).37 It can be seen that the chemical valence of cerium on the surface of the samples was a mixed valence state, and was mainly Ce4+ plus a small fraction of Ce3+. The semi-quantified calculations of the amount of Ce3+ were following the equation Ce3+ = [Av0 + Av′+ Au0 + Au′]/[Av0 + Av + Av′ + Av′′ + Av′′ + Au0 + Au + Au′ + Au′′ + Au′′′], and the values were 30.1% (CeO2-PH) and 17.6% (CeO2-NP), respectively. The high content of Ce3+ in CeO2-PH was ascribed to the solvent (ethylene glycol), smaller primary particle size,29 and more oxygen vacancy defects. Ethylene glycol has reducibility and Ce4+ can be converted into Ce3+ in the reaction process. In the case of more oxygen vacancy defects, proved in Raman spectra (Fig. 6), more oxygen vacancy defects facilitated more amount of Ce3+. The reason why CeO2-PH (100) can generate a large amount of Ce3+ compared with CeO2-NP (111) is associated with the exposed crystal planes. The oxygen vacancy formation energy, nature and amount of the defects and low coordination sites are intrinsically affected by the surface planes of the ceria nanoshapes. Based on density functional theory calculations, the stability follows the sequence (111) > (110) > (100), while the activity follows the opposite order. The energy required to form oxygen vacancies on the (100) surface is less than those on the (111) and (110) surfaces due to its intrinsic high energy.
Photoelectrochemical response
In order to further account for the high activity of CeO2-PH, its photoelectrochemical response has been measured.38–40 The photoelectrochemical properties of the two types of CeO2 were characterized by measuring the photocurrent under a 500 W Xe lamp equipped with a cutoff filter (λ > 325 nm), and the initial and final potential were 0 V and 0.4 V, respectively. Fig. 8 shows the photocurrent responses of the photoanodes prepared from CeO2-PH and CeO2-NP. As anticipated, CeO2-PH exhibited an excellent photoelectrochemical response, being about 5-times higher than that of CeO2-NP. The enhancement of the photocurrent for CeO2-PH can be attributed to the improvement of light-harvesting, as shown in the result for band gap value. In addition, the high content of Ce3+ ions would be trapped by the photogeneration holes,32 and then facilitated the separation of the photogeneration electron–hole pairs. The trapped holes would restrain the recombination of the photogeneration electron–hole, and then enhanced the photocurrent response. As a result, CeO2-PH (higher Ce3+ ion content) has a stronger photoelectrochemical response, which is in accordance with activity in the evaluation of acetaldehyde degradation. Moreover, the defects, shown in Raman and XPS characterizations, in CeO2-PH might lead to the formation of a surface state energy band of oxygen and the oxygen adsorption, desorption and diffusion processes would easily occur on the surface, resulting in notable changes in the properties of CeO2-PH such as optical and electrical properties.41 When the incident light is larger than the band gap, previously adsorbed oxygen on the surface of the CeO2-PH will desorb and release free electrons, causing the conductivity to increase. | ||
| Fig. 8 Photocurrent response of the photoanodes to light on–off collected from CeO2-NP and CeO2-PH electrodes in the solution of 1.0 M Na2SO4 under light illumination. | ||
Conclusion
In summary, porous CeO2 hollow spheres were successfully synthesized by a one-step solvothermal method in the absence of any templates. Based on the morphology evolution of that time-dependent samples (Fig. S1†), it is thought that Ostwald ripening occurs and that it is the main driving force for the core evacuation of solid aggregates during the hollowing process. Furthermore, the prepared CeO2-PH has high activity for decomposition of the acetaldehyde. The presence of Ce3+ and oxygen vacancies in CeO2-PH enhanced the light harvesting and provided activity sites in the photocatalytic process. In addition, this prepared special porous-hollow sphere can be used in many potential applications in the future.Acknowledgements
This work was supported by JST PRESTO program and JST ACT-C program. The authors are also grateful for financial support by the National Natural Science Foundation of China (no. 50873085 and no. 21307104).References
- P. M. Arnal, M. Comotti and F. Schuth, Angew. Chem., 2006, 45, 8224–8227 CrossRef CAS PubMed.
- L. Liao, H. X. Mai, Q. Yuan, H. B. Lu, J. C. Li, C. Liu, C. H. Yan, Z. X. Shen and T. Yu, J. Phys. Chem. C, 2008, 112, 9061–9065 CAS.
- X. Liang, J. J. Xiao, B. H. Chen and Y. D. Li, Inorg. Chem., 2010, 49, 8188–8190 CrossRef CAS PubMed.
- Y. F. Zhu, J. L. Shi, W. H. Shen, X. P. Dong, J. W. Feng, M. L. Ruan and Y. S. Li, Angew. Chem., Int. Ed., 2005, 44, 5083–5087 CrossRef CAS PubMed.
- H. Ma, F. Cheng, J. Chen, J. Zhao, C. Li, Z. Tao and J. Liang, Adv. Mater., 2007, 19, 4067–4070 CrossRef CAS.
- B. Xu, B. Huang, H. Cheng, Z. Wang, X. Qin, X. Zhang and Y. Dai, Chem. Commun., 2012, 48, 6529–6531 RSC.
- Z. Yang, J. Wei, H. Yang, L. Liu, H. Liang and Y. Yang, Eur. J. Inorg. Chem., 2010, 2010, 3354–3359 CrossRef.
- C. Laberty-Robert, J. W. Long, E. M. Lucas, K. A. Pettigrew, R. M. Stroud, M. S. Doescher and D. R. Rolison, Chem. Mater., 2006, 18, 50–58 CrossRef CAS.
- D. Zhang, X. Du, L. Shi and R. Gao, Dalton Trans., 2012, 41, 14455–14475 RSC.
- D. Gerceker and I. Onal, Appl. Surf. Sci., 2013, 285, 927–936 CrossRef CAS PubMed.
- K. Tanimoto, H. Kato, S. Takeshita, M. Hidaka, S. Hinokuma and M. Machida, Bull. Chem. Soc. Jpn., 2013, 86, 1327–1332 CrossRef CAS.
- Z. Zhan, X. Liu, D. Ma, L. Song, J. Li, H. He and H. Dai, Front. Environ. Sci. Eng., 2014, 8, 483–495 CrossRef CAS.
- C. Ho, J. C. Yu, T. Kwong, A. C. Mak and S. Lai, Chem. Mater., 2005, 17, 4514–4522 CrossRef CAS.
- S. W. Yang and L. Gao, J. Am. Chem. Soc., 2006, 128, 9330–9331 CrossRef CAS PubMed.
- Q. Wu, F. Zhang, P. Xiao, H. Tao, X. Wang, Z. Hu and Y. Lü, J. Phys. Chem. C, 2008, 112, 17076–17080 CAS.
- T. Taniguchi, K.-i. Katsumata, S. Omata, K. Okada and N. Matsushita, Cryst. Growth Des., 2011, 11, 3754–3760 CAS.
- N. C. Strandwitz and G. D. Stucky, Chem. Mater., 2009, 21, 4577–4582 CrossRef CAS.
- G. Chen, C. Xu, X. Song, S. Xu, Y. Ding and S. Sun, Cryst. Growth Des., 2008, 8, 4449–4453 CAS.
- X. Liang, X. Wang, Y. Zhuang, B. Xu, S. Kuang and Y. Li, J. Am. Chem. Soc., 2008, 130, 2736–2737 CrossRef CAS PubMed.
- C. Zhang, X. Zhang, Y. Wang, S. Xie, Y. Liu, X. Lu and Y. Tong, New J. Chem., 2014, 38, 2581–2586 RSC.
- B. Xu, Q. Zhang, S. Yuan, M. Zhang and T. Ohno, Appl. Catal., B, 2015, 164, 120–127 CrossRef CAS PubMed.
- E. Aneggi, D. Wiater, C. de Leitenburg, J. Llorca and A. Trovarelli, ACS Catal., 2014, 4, 172–181 CrossRef CAS.
- Y. Chen, S. Lv, C. Chen, C. Qiu, X. Fan and Z. Wang, J. Phys. Chem. C, 2014, 118, 4437–4443 CAS.
- P. Zhao, A. Ito and T. Goto, Ceram. Int., 2014, 40, 605–609 CrossRef CAS PubMed.
- H. C. Zeng, Curr. Nanosci., 2007, 3, 177–181 CrossRef CAS.
- F. Z. Guozhu Chen, X. Sun, S. Sun and R. Chen, CrystEngComm, 2011, 13, 2904–2908 RSC.
- J. C. Groen, L. A. A. Peffer and J. Pérez-Ramírez, Microporous Mesoporous Mater., 2003, 60, 1–17 CrossRef CAS.
- Z. Wang, Z. Quan and J. Lin, Inorg. Chem., 2007, 46, 5237–5242 CrossRef CAS PubMed.
- S. Deshpande, S. Patil, S. V. N. T. Kuchibhatla and S. Seal, Appl. Phys. Lett., 2005, 87, 133113 CrossRef PubMed.
- M. Y. Chen, X. T. Zu, X. Xiang and H. L. Zhang, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 389, 263–268 CrossRef CAS PubMed.
- S. Tsunekawa, J.-T. Wang, Y. Kawazoe and A. Kasuya, J. Appl. Phys., 2003, 94, 3654 CrossRef CAS PubMed.
- X. Lu, T. Zhai, H. Cui, J. Shi, S. Xie, Y. Huang, C. Liang and Y. Tong, J. Mater. Chem., 2011, 21, 5569 RSC.
- J. Lin, L. Li, Y. Huang, W. Zhang, X. Wang, A. Wang and T. Zhang, J. Phys. Chem. C, 2011, 115, 16509–16517 CAS.
- B. Liu, M. Yao, B. Liu, Z. Li, R. Liu, Q. Li, D. Li, B. Zou, T. Cui, G. Zou, J. Liu and Z. Chen, J. Phys. Chem. C, 2011, 115, 4546–4551 CAS.
- J. E. Spanier, R. D. Robinson, Z. Feng, C. Siu-Wai and I. P. Herman, Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 64, 245407 CrossRef.
- X. W. Liu, K. B. Zhou, L. Wang, B. Y. Wang and Y. D. Li, J. Am. Chem. Soc., 2009, 131, 3140–3141 CrossRef CAS PubMed.
- E. Bêche, P. Charvin, D. Perarnau, S. Abanades and G. Flamant, Surf. Interface Anal., 2008, 40, 264–267 CrossRef.
- A. Paracchino, V. Laporte, K. Sivula, M. Grätzel and E. Thimsen, Nat. Mater., 2011, 10, 456–461 CrossRef CAS PubMed.
- Y. Liu, S. Xie, C. Liu, J. Li, X. Lu and Y. Tong, J. Power Sources, 2014, 269, 98–103 CrossRef CAS PubMed.
- G. L. Wang, K. L. Liu, Y. M. Dong, X. M. Wu, Z. J. Li and C. Zhang, Biosens. Bioelectron., 2014, 62, 66–72 CrossRef CAS PubMed.
- Y.-W. Zhang, R. Si, C.-S. Liao, C.-H. Yan, C.-X. Xiao and Y. Kou, J. Phys. Chem. B, 2003, 107, 10159–10167 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra12127a |
| This journal is © The Royal Society of Chemistry 2014 |

