Flexible, micro-porous chitosan–gelatin hydrogel/nanofibrin composite bandages for treating burn wounds
Abstract
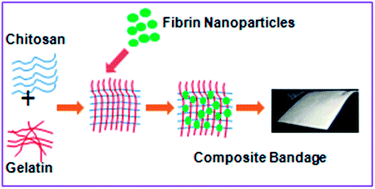
We developed chitosan–gelatin hydrogel/nanofibrin ternary composite bandages for the treatment of burn wounds and characterized the material by SEM. The spherical nanofibrin moieties (229 ± 3 nm in size) were prepared using an emulsification method and were distributed within the chitosan–gelatin matrix. The presence of the fibrin component within the matrix was confirmed by SEM and phosphotungstic acid-hematoxylin staining. The swelling, biodegradation, porosity, whole-blood clotting, platelet activation, cell viability, cell attachment and cell infiltration properties of the nanocomposite bandages were evaluated. The nanocomposite bandages were flexible, degradable and showed enhanced blood clotting and platelet activity compared with control samples. The nanocomposite bandages showed adequate swelling ability when immersed in water and phosphate-buffered saline. Cell viability studies on normal human dermal fibroblast and human umbilical cord vein endothelial cells proved the non-toxic nature of the composite bandages. Cell attachment and infiltration studies showed that the human dermal fibroblast and human umbilical cord vein endothelial cells attached to the bandage. Enhanced collagen deposition and re-epithelialization with the formation of intact mature epidermis was noted in the animal groups treated with the nanocomposite bandages compared with the experimental controls. These results show that these ternary nanocomposite bandages are ideal candidates for burn wound dressings.

 Please wait while we load your content...
Please wait while we load your content...